Editor: Sarah
Radiation colitis, a condition caused by pelvic radiotherapy, is a significant source of discomfort for patients, resulting in symptoms like inflammation, diarrhea, and bleeding in the colon. This condition has long been linked to cellular damage induced by radiation, primarily through oxidative stress. A recent study presents an oral drug delivery system that specifically targets ferroptosis, a form of cell death associated with radiation-induced intestinal injury. This new treatment, utilizing ceria (CeO2) anchored on halloysite nanotubes (HNTs) and combined with an iron chelator, offers a potential method for alleviating radiation colitis and improving the quality of life for radiotherapy patients.
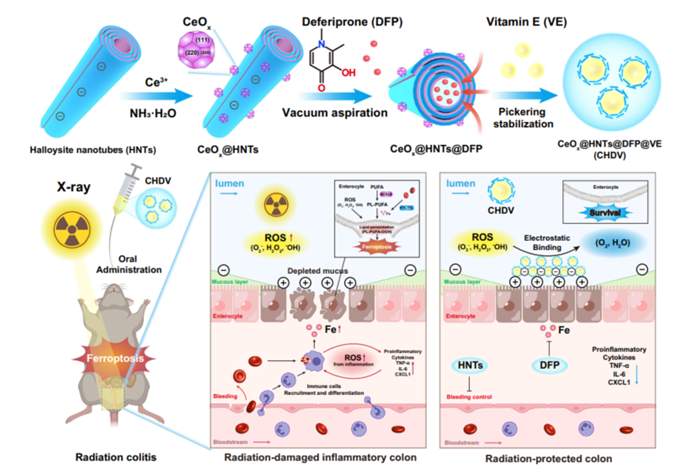
Figure 1: Schematic representation of the CeO2@HNTs@DFP Pickering emulsion.
Contribution to Radiation Protection Therapy
This study represents a shift in focus from apoptosis to ferroptosis as a key factor in radiation colitis. Ferroptosis, a regulated cell death pathway driven by oxidative stress and iron overload, has been identified as a critical component in the pathogenesis of radiation colitis. The researchers have developed an innovative approach to mitigate damage caused by pelvic radiotherapy by targeting this form of cell death.
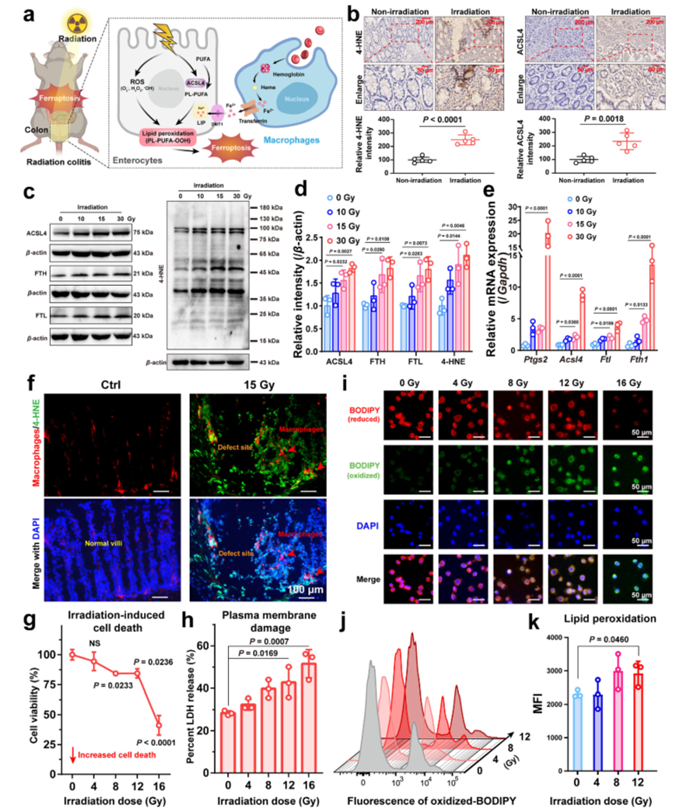
Figure 2: In vivo therapeutic effect of CeO2@HNTs@DFP in mice with radiation colitis.
Key Findings
- Ferroptosis as a Key Contributor to Radiation Colitis: The study highlights the central role of ferroptosis in radiation-induced intestinal injury. Elevated levels of markers of lipid peroxidation and ferroptosis-related proteins were observed in the colon tissue of irradiated animals, indicating that ferroptosis contributes significantly to colitis development.
- Development of a Novel Nanoplatform for Therapy: The team created a CeO2@HNTs@DFP oral nanoplatform that combines ceria nanoparticles, which scavenge reactive oxygen species (ROS), with deferiprone (DFP), an iron chelator. This combination was shown to alleviate iron overload and reduce ferroptosis, providing protection against radiation-induced colitis. The nanoplatform demonstrated significant efficacy in preclinical models, reducing symptoms like diarrhea and hematochezia while improving survival rates.
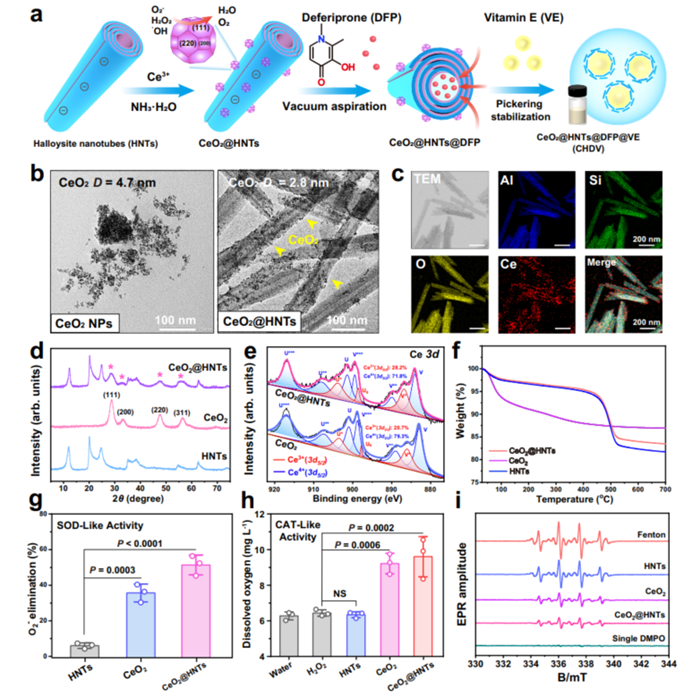
Figure 3: The CeO2@HNTs@DFP nanoplatform structure and its mechanism for drug delivery.
- In Vivo Effectiveness: In mouse models, the CeO2@HNTs@DFP nanoplatform significantly alleviated common symptoms of radiation colitis, including diarrhea and blood in the stool. Furthermore, the treatment increased survival rates in the irradiated mice, suggesting that the nanoplatform has considerable potential for clinical application in patients undergoing pelvic radiotherapy.
Methodology
The researchers designed a Pickering emulsion stabilized by HNTs, modified with ceria nanoparticles and loaded with DFP. This formulation was tested both in vitro and in vivo. The emulsion demonstrated excellent stability, effective ROS scavenging properties, and targeted delivery to the inflamed colon tissue. The electrostatic interactions between the charged HNTs and the inflamed tissue ensured precise drug delivery to the damaged area, optimizing therapeutic effects.
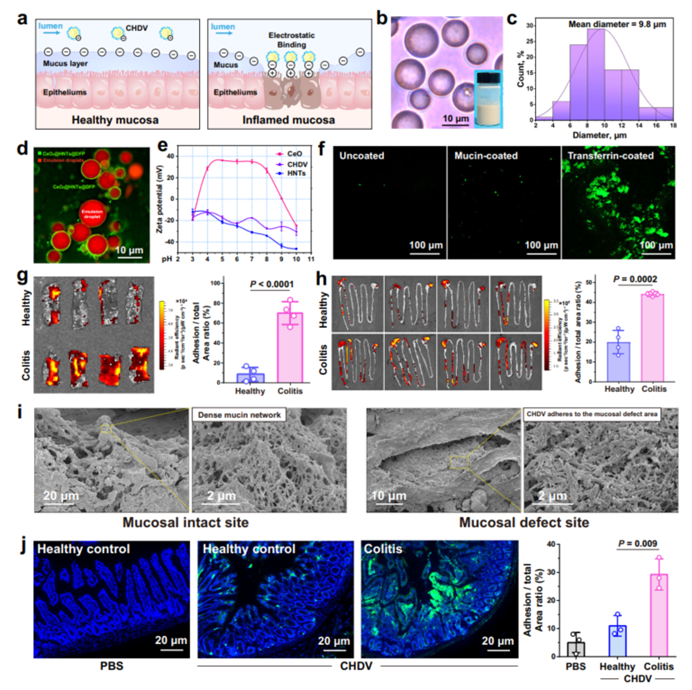
Figure 4: Targeted delivery of CeO2@HNTs@DFP to inflamed colon tissue.
Implications for Radiation Therapy and Broader Applications
This research could mark a significant advancement in the treatment of radiation colitis. By focusing on ferroptosis and oxidative stress, the study introduces new therapeutic strategies for patients undergoing radiation therapy, potentially reducing the side effects typically associated with radiotherapy. The findings suggest that this approach may also be applicable to other radiation-induced tissue damage, offering broader implications for the treatment of patients with radiation injuries.
Conclusion
The development of the CeO2@HNTs@DFP oral nanoplatform offers a promising new approach to alleviating radiation colitis. By targeting ferroptosis and oxidative stress, the study introduces a novel therapeutic strategy that could improve outcomes for patients undergoing pelvic radiotherapy. With successful results in animal models, this treatment has the potential to transform the management of radiation-induced intestinal damage, providing hope for better therapeutic outcomes in the future.
Reference
Feng, Yue, et al. “A Ferroptosis-Targeting Ceria Anchored Halloysite as an Oral Drug Delivery System for Radiation Colitis Therapy.” Nature Communications, vol. 14, no. 5083, 2023, https://doi.org/10.1038/s41467-023-40794-w.
