Editor: Nina
Scientists develop chitosan-coated silicon nanoparticles to enhance tumor-targeting and biodistribution in glioblastoma therapy, paving the way for more effective and biocompatible cancer treatments.
Key Preview
- Research Question: Can chitosan-coated ultrapure silicon nanoparticles (SiNPs-CH) enhance the biodistribution and tumor-targeting efficacy of silicon nanoparticles in cancer therapy?
- Research Design and Strategy: This study utilized in vivo experiments on nude mice with glioblastoma to evaluate the biodistribution, toxicity, and therapeutic potential of SiNPs-CH.
- Method: Researchers synthesized SiNPs using femtosecond laser ablation, coated them with chitosan, and analyzed their biodistribution following intravenous administration.
- Key Results: SiNPs-CH displayed prolonged circulation time in the bloodstream, improved accumulation in tumor sites, and no significant toxicity was observed in major organs.
- Significance of the Research: This study highlights the potential of SiNPs-CH as a promising tumor-targeting nanosystem, paving the way for advanced cancer therapies.
Introduction
Cancer remains one of the most significant global health challenges, with glioblastoma being one of the most aggressive and common forms of brain cancer. Characterized by rapid growth and a tendency to invade surrounding brain tissue, glioblastoma presents a formidable barrier to effective treatment. Patients diagnosed with this malignancy often face poor prognosis, with survival rates remaining low despite advances in surgical, radiotherapy, and chemotherapy techniques.
Traditional treatment strategies for glioblastoma typically involve a combination of surgery, radiotherapy, and systemic chemotherapy. The primary goal of these approaches is to remove as much of the tumor as possible while also targeting residual cancer cells. However, a common challenge with these conventional methods is the limited ability to deliver therapeutic agents directly to the tumor site. This limitation arises from the presence of the blood-brain barrier, which restricts the passage of most drugs into the central nervous system. Consequently, many patients experience suboptimal drug concentrations at the tumor site, leading to inadequate therapeutic responses and an increased risk of side effects from systemic exposure.
The challenges faced in current glioblastoma treatment strategies result in a pressing need for more effective drug delivery systems. Conventional therapies often fail to achieve sufficient drug accumulation within tumors, leading to treatment resistance and tumor recurrence. Furthermore, the toxicity of systemic therapies can severely impact the patient’s quality of life, highlighting the necessity for targeted approaches that minimize off-target effects.
In response to these challenges, innovative drug delivery strategies utilizing nanotechnology have emerged as promising alternatives. One such strategy involves the use of silicon nanoparticles (SiNPs) functionalized with biocompatible polymers, such as chitosan. This approach aims to enhance the biodistribution and accumulation of therapeutic agents directly at tumor sites, leveraging the enhanced permeability and retention (EPR) effect. By improving the stability and circulation time of nanoparticles in the bloodstream, this innovative delivery system seeks to overcome the barriers posed by the blood-brain barrier, ultimately leading to more effective and targeted treatments for glioblastoma and other malignancies.
Research Team and Aim
The research team was led by Tarek Baati, alongside co-authors Imen Chaabani, Abir Salek, Leila Njim, Mouna Selmi, Ahmed Al-Kattan, and Karim Hosni. This collaborative effort took place at the Institut National de Recherche et d’Analyse Physico-chimique in Tunisia and was conducted in 2023. The findings of this study were published in the journal Nanoscale Advances under the title “Chitosan-coated ultrapure silicon nanoparticles produced by laser ablation: biomedical potential in nano-oncology as a tumor-targeting nanosystem.”
The aim of the research, as articulated by lead researcher Tarek Baati, was to enhance the therapeutic properties of silicon nanoparticles in cancer therapy by functionalizing them with chitosan, thereby improving their biodistribution and tumor-targeting capabilities. This innovative approach seeks to address the limitations of traditional drug delivery methods, particularly in the context of glioblastoma treatment.
Experimental Process
Primary Technique
The primary technique employed in this research was the synthesis and functionalization of ultrapure silicon nanoparticles (SiNPs) using femtosecond laser ablation in water. This approach allowed for the production of high-purity nanoparticles without toxic residues, establishing a foundation for their potential use in biomedical applications, particularly in cancer therapy.
Synthesis of SiNPs
- Preparation: A silicon wafer was immersed in 20 mL of deionized water contained in a chamber.
- Laser Ablation: A Yb:KGW femtosecond laser (1025 nm, 480 fs, 500 mJ, 1–5 kHz) was focused on the silicon wafer’s surface using a 75 mm lens to initiate the ablation process.
- Recovery: After synthesis, the resulting SiNPs were centrifuged at 45,000 rpm for 25 minutes using a Beckman ultracentrifuge to recover the nanoparticles for subsequent modifications.
Coating of SiNPs with Chitosan
- Chitosan Solution Preparation: Chitosan (75% deacetylated) was dissolved in 1 wt% acetic acid, creating a 0.15 g chitosan solution in 5 mL of the acidic medium.
- Mixing: Fifty milligrams of ultrapure SiNPs were dispersed in the chitosan solution and subjected to ultrasonic agitation for uniform distribution.
- Heating: The mixture was stirred at 60 °C for 6 hours to facilitate the coating process, followed by cooling in an ice bath for one hour.
- Washing: The chitosan-coated nanoparticles (SiNPs-CH) were washed multiple times with deionized water to achieve a neutral pH, ensuring excess chitosan was removed before drying overnight at 50 °C.
Characterization of SiNPs-CH
- Transmission Electron Microscopy (TEM): The morphology and size of SiNPs-CH were assessed using a Tecnai G2 transmission electron microscope at 200 kV. Images were captured to analyze particle shapes and size distribution.
- Dynamic Light Scattering (DLS): The hydrodynamic size and zeta potential of the nanoparticles were determined using a Zetasizer Nano ZS instrument. Samples were sonicated for 5 minutes before measurements were taken to ensure homogeneity.
- Fourier-transform Infrared Spectroscopy (FTIR): The chemical composition and surface functionalization of SiNPs-CH were analyzed using FTIR within the range of 500-4000 cm^-1 to confirm the presence of chitosan on the nanoparticle surface.
- X-ray Diffraction (XRD): XRD patterns were obtained to characterize the crystallinity of SiNPs and verify the presence of SiO2.
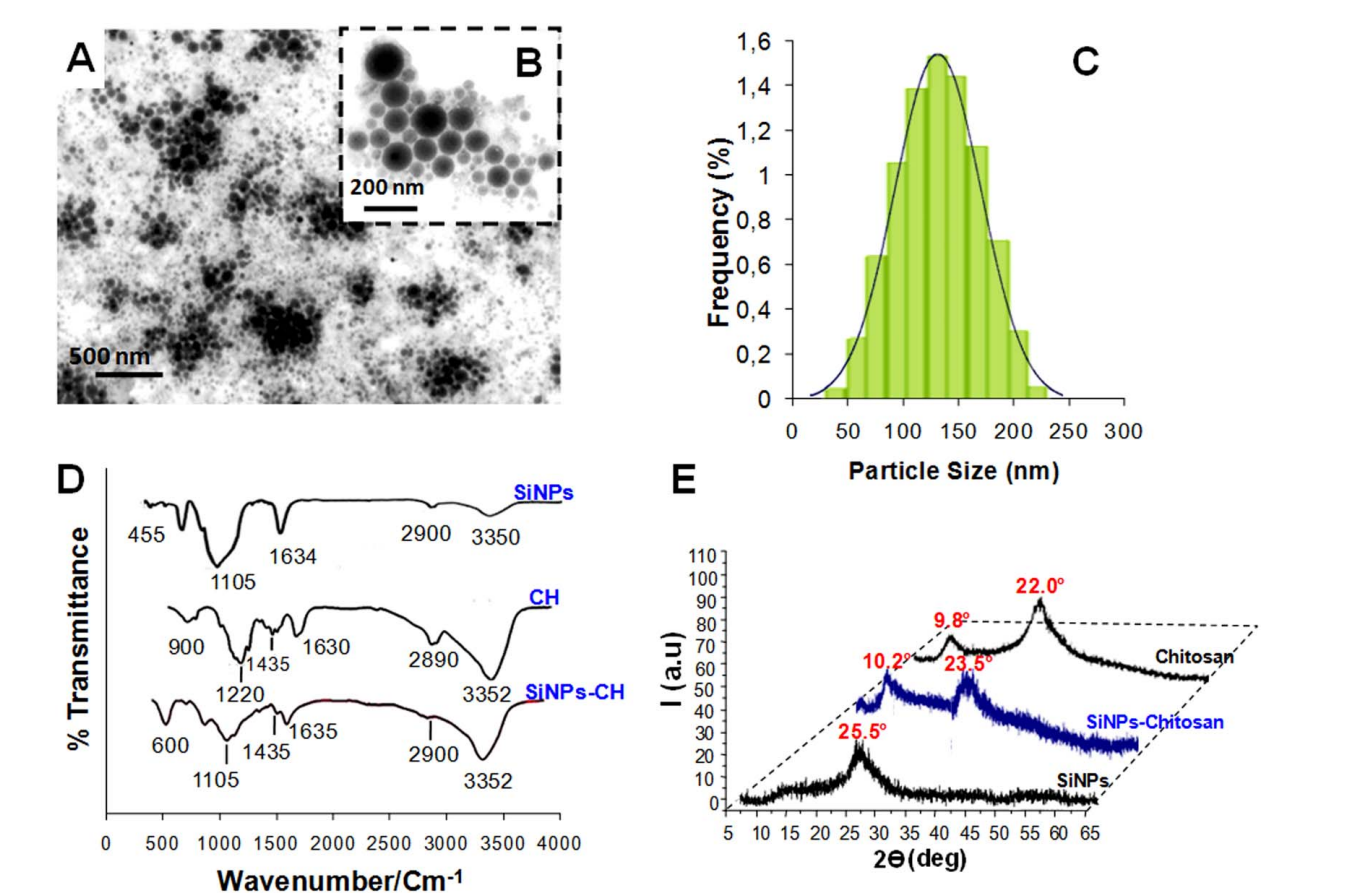
Figure 1. (A and B) TEM images of SINPs-CH nanoparticles produced by laser ablation in water and then modified by chitosan (CH). (B) is a magnification of (A). © Particle-size distribution of the chitosan-coated nanoparticles (SiNPs-CH) determined by a Zetasizer Nano ZS instrument (DLS). (D) FTIR spectra of chitosan, SiNPs, and SiNPs-CH. (E) XRD patterns of SiNPs-CH compared to those of chitosan and uncoated SiNPs.
In Vivo Experiments
- Animal Model Preparation: Thirty male athymic nude mice were acclimatized for seven days before subcutaneously injecting them with U87-MG glioma cells (1 × 10^7 cells per mouse) to develop solid tumors.
- Treatment Administration: After 14 days and tumor sizes averaging 100 mm^3, the mice were divided into groups and injected intravenously with a single dose of 20 mg kg^-1 of SiNPs-CH.
- Biodistribution Assessment: Mice were sacrificed at 1, 7, and 15 days post-injection. Blood, urine, feces, and major organs (liver, spleen, kidneys, heart, lungs, brain) were harvested for silicon content analysis using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES).
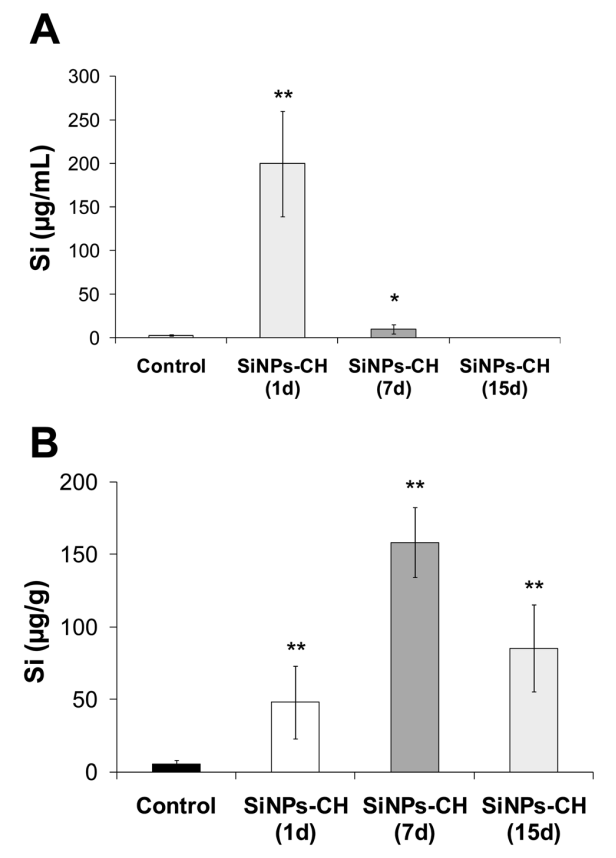
Figure 2. Silicon concentration determined by ICP-OES in serum and tumor tissue at 1, 7, and 15 days following the intravenous administration of SiNPs-CH (20 mg kg−1 ). (A) Silicon concentration in serum. (B) Silicon concentration in the tumor. Results are representative of 6 animals (mean ± SD, n = 6). Statistical significance was determined by Tukey HSD test (*p < 0.05, **p < 0.01).
Data Collection and Analysis
Data were collected through various means, including serum, urine, feces, and tissue samples analyzed for silicon concentration. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS), with results presented as mean ± standard deviation. Comparisons with control groups were made using the Tukey HSD test, ensuring rigor in evaluating the significance of findings.
Novel Aspects
This study introduced the novel approach of coating SiNPs with chitosan, significantly enhancing their colloidal stability, circulation time, and tumor-targeting capabilities compared to traditional nano-delivery systems. The use of femtosecond laser ablation for nanoparticle synthesis ensured high purity and biocompatibility, while the chitosan coating facilitated prolonged bloodstream presence and targeted accumulation in tumor environments through the enhanced permeability and retention (EPR) effect. Overall, this work provides a promising framework for developing biodegradable, non-toxic nanoparticles for effective cancer therapy.
Conclusion
The successful development of the chitosan-coated silicon nanoparticle (SiNPs-CH) drug delivery system was achieved through a series of carefully designed experiments that demonstrated its enhanced biodistribution and tumor-targeting capabilities in a glioblastoma model. By utilizing femtosecond laser ablation to synthesize ultrapure silicon nanoparticles and subsequently functionalizing them with chitosan, the researchers effectively improved the nanoparticles’ stability, circulation time, and ability to accumulate in tumor tissues.
The highlights of the study include the significant increase in the circulation time of SiNPs-CH in the bloodstream, allowing for prolonged exposure and enhanced accumulation in tumors via the enhanced permeability and retention (EPR) effect. Importantly, the research confirmed the biocompatibility of the SiNPs-CH, with no observed toxicity in major organs, thereby paving the way for their potential use as a targeted drug delivery system in cancer therapy. Overall, the findings underscore the promise of SiNPs-CH as an innovative approach in the field of nano-oncology, offering a pathway to more effective and less toxic cancer treatments.
Reference:
Baati, Tarek, et al. “Chitosan-coated ultrapure silicon nanoparticles produced by laser ablation: biomedical potential in nano-oncology as a tumor-targeting nanosystem.” Nanoscale Advances 5.11 (2023): 3044-3052.
