Editor: Nina
Scientists develop membrane-camouflaged nanoparticles using red blood cell membranes to effectively neutralize Clostridium perfringens ε-toxin, providing a novel therapeutic approach for toxin-induced infections.
Key Preview
Research Question
Can membrane-camouflaged nanoparticles (MNPs) effectively neutralize Clostridium perfringens ε-toxin (ETX) and provide safe therapeutic options for toxin-induced infections?
Research Design and Strategy
This study systematically evaluated the safety and therapeutic efficacy of MNPs, which are designed using membranes from different cell origins, to neutralize ETX in vitro and in vivo.
Method
The researchers prepared MNPs using membranes from red blood cells (RBCs) and Madin-Darby canine kidney (MDCK) cells, assessing their neutralization capacity against ETX through cytotoxicity assays and animal models.
Key Results
MNPs demonstrated significant safety and efficacy in neutralizing ETX, with RBC-derived MNPs showing superior protective effects and lower immunogenicity compared to MDCK-derived MNPs. Both intravenous injection and nebulized inhalation of RBC-NPs effectively protected mice against ETX intoxication.
Significance of the Research
This study provides promising insights into the use of MNPs as a novel therapeutic approach to treat ETX infections, highlighting the importance of membrane origin and delivery routes in enhancing nanoparticle efficacy and safety.
Introduction
Clostridium perfringens ε-toxin (ETX) is a potent exotoxin primarily associated with enterotoxemia, a rapidly fatal disease that affects livestock, particularly sheep and goats. This toxin is produced by C. perfringens types B and D and is a significant virulence factor responsible for severe economic losses in the agricultural sector. Although ETX poses a lesser-known threat to human health, it can inflict damage on red blood cells and other vital organs, raising concerns about its potential use as a biological weapon. Despite the gravity of ETX-related infections, effective therapeutic options for treating this toxin remain elusive.
Traditional treatment strategies for toxin-induced diseases often involve the administration of antitoxins or antibiotics. These conventional approaches primarily focus on neutralizing the toxin or eradicating the bacteria responsible for its production. Drug delivery methods typically include intravenous injection or oral administration; however, these methods present several challenges. For instance, intravenous delivery can lead to rapid clearance of the therapeutic agents from the bloodstream, limiting their efficacy. Additionally, traditional methods may induce significant systemic side effects or fail to effectively target the affected tissues, resulting in suboptimal therapeutic outcomes.
The challenges presented by current treatment approaches highlight the need for more effective and targeted drug delivery systems. To address these issues, the study explores an innovative strategy involving membrane-camouflaged nanoparticles (MNPs). This novel approach utilizes cell membranes derived from red blood cells (RBCs) to encapsulate therapeutic nanoparticles, thereby enhancing their biocompatibility and prolonging their circulation time in the bloodstream. By mimicking the natural properties of RBC membranes, these MNPs can significantly improve the targeted delivery of therapeutics, reduce immunogenicity, and enhance the neutralization of ETX. This innovative drug delivery system represents a promising advancement in the treatment of toxin-induced infections, with the potential for broader applications in various medical conditions.
Research Team and Aim
The research team behind this study comprises scholars from Hebei Normal University and the Academy of Military Medical Sciences. The team is led by Dr. Jinglin Xu, who conducted this research in collaboration with co-researchers Dongxue Li, Lin Kang, Tingting Liu, and others. Their findings were published in the paper titled “Systematic evaluation of membrane-camouflaged nanoparticles in neutralizing Clostridium perfringens ε-toxin,” which appeared in the Journal of Nanobiotechnology.
The primary aim of the research, as articulated by Dr. Xu, is to evaluate the safety and therapeutic efficacy of membrane-camouflaged nanoparticles (MNPs) derived from red blood cells and Madin-Darby canine kidney (MDCK) cell membranes in neutralizing ETX. This study seeks to contribute to the development of effective clinical treatments for infections caused by this potent toxin, ultimately addressing the urgent need for innovative therapeutic strategies in the face of traditional treatment limitations.
Experimental Process
Primary Technique
The primary technique employed in this study was the preparation and characterization of membrane-camouflaged nanoparticles (MNPs) using an innovative biomimetic approach. This method involved the use of cell membranes from red blood cells (RBCs) and Madin-Darby canine kidney (MDCK) cells to create nanoparticles designed to neutralize Clostridium perfringens ε-toxin (ETX) effectively and safely.
Nanoparticle Preparation
- Nanoparticle Fabrication: PLGA nanoparticles were synthesized using an emulsion-evaporation method. Carboxy-terminated PLGA was dissolved in dichloromethane (DCM) and mixed with polyvinyl alcohol (PVA) in aqueous solution to form a stable emulsion. This mixture was then stirred for 4 hours in a fume hood at 25°C to allow solvent evaporation and nanoparticle formation.
- Membrane Extraction: RBCs were isolated by centrifugation of whole blood at 1,000 × g for 10 minutes, followed by washing three times with phosphate-buffered saline (PBS) to remove plasma. The RBCs were then subjected to osmotic lysis to obtain cell membranes, which were further washed to eliminate residual hemoglobin.
- Coating Technique: The extracted cell membranes were mixed with the PLGA nanoparticles and extruded through 100 nm polycarbonate membranes to form RBC-NPs and MDCK-NPs. This mechanical squeezing method allowed for effective membrane coating on the nanoparticles.
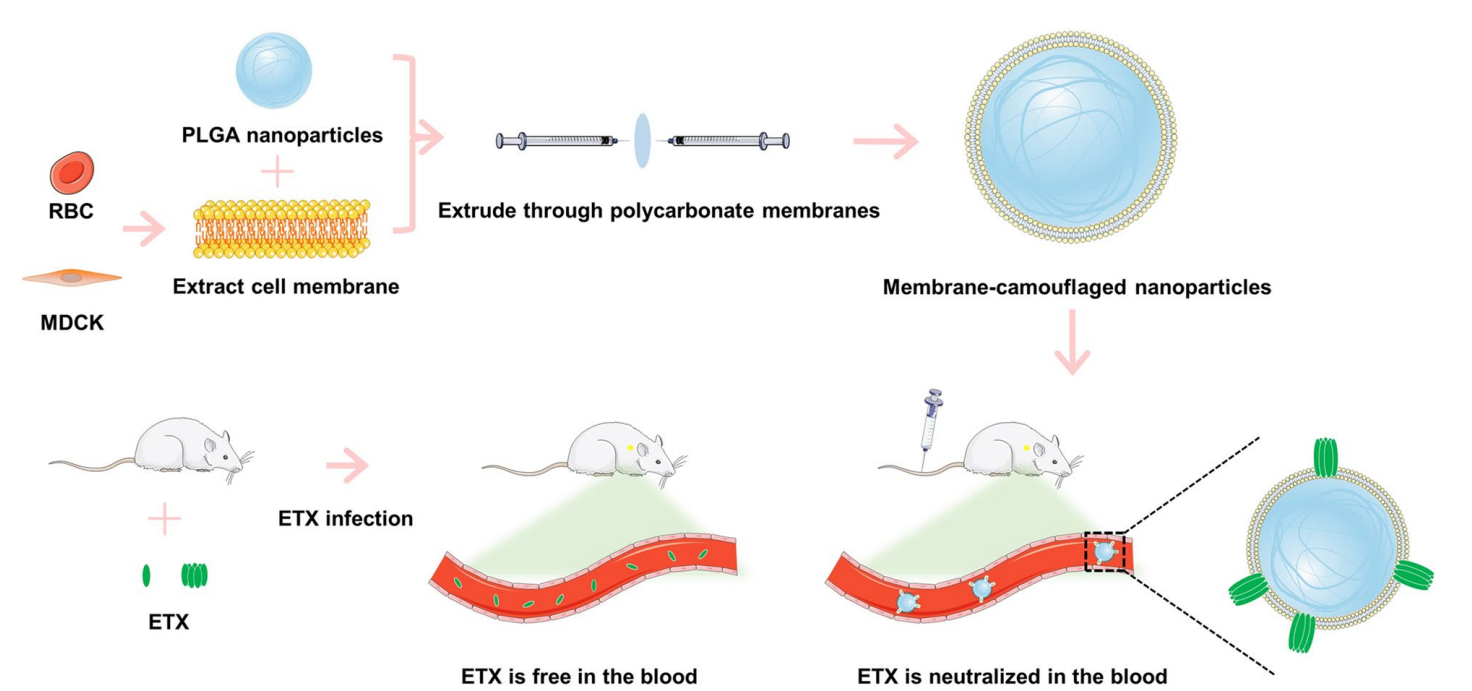
Figure 1. Schematic illustration of the procedure used to prepare RBC membrane-camoufaged nanoparticles (RBC-NPs) or MDCK cell membrane-camoufaged nanoparticles (MDCK-NPs). Membrane-camoufaged NPs can neutralize ETX in mice infected with ETX.
In Vitro Evaluation
- Cytotoxicity Assays: To evaluate the neutralization capacity of MNPs, MDCK cells were seeded in 96-well plates and treated with varying concentrations of GST-ETX. Following a 1-hour incubation, the culture medium was replaced, and MTS reagent was added to assess cell viability. The concentration causing 50% cell death (CT50) was determined through absorbance measurements at 492 nm.
- Neutralization Tests: The ability of both RBC-NPs and MDCK-NPs to neutralize ETX was tested by incubating the nanoparticles with a fixed concentration of GST-ETX and measuring the resulting cell viability in MDCK cells. A comparative analysis of the protective effects of each nanoparticle type was conducted to assess their efficacy.
Figure 2.In vitro evaluation of GST-ETX and MNPs
In Vivo Safety Assessment
- Animal Model: Eight-week-old female BALB/c mice were used for the safety evaluation of the MNPs. Mice were divided into groups and injected intravenously with either PBS, bare nanoparticles, RBC-NPs, or MDCK-NPs.
- Monitoring and Analysis: Mice were observed for 7 days post-injection for survival and any adverse effects. Blood samples were collected to analyze various parameters, including white blood cell (WBC) counts and other serum markers indicative of liver and kidney function. Statistical analyses were performed to compare the results among different treatment groups.
Figure 3. In vivo safety evaluations of the three kinds of the nanoparticles
In Vivo Therapeutic Efficacy Evaluations
- Efficacy Testing: Mice were injected with a lethal dose of GST-ETX (20 ng) followed by intravenous administration of RBC-NPs. Survival rates were monitored over a period of 7 days, and histopathological analysis of major organs was conducted post-mortem to assess organ damage.
- Interaction Studies: Fluorescently labeled RBC-NPs and GST-ETX were injected into mice to study their interaction and metabolism in vivo. The distribution of the nanoparticles and the toxin in blood and organs was tracked using fluorescence imaging at multiple time points (5 min, 24 h, 48 h, and 72 h).
Figure 4.In vivo therapeutic efcacy evaluations of the RBC-NPs
Data Collection and Analysis
Data were collected through a combination of fluorescence imaging for in vivo studies and quantitative assays for in vitro evaluations. The results from cytotoxicity assays were statistically analyzed using appropriate tests (e.g., Student’s t-tests and ANOVAs) to determine significant differences among treatment groups. Fluorescence intensity measurements provided insights into the biodistribution and metabolism of the nanoparticles and toxins.
Novel Aspects
The innovative aspect of this study lies in the use of RBC membranes for camouflaging nanoparticles, which significantly reduced immunogenicity compared to karyocyte-derived membranes. This approach not only enhanced the safety profile of the therapeutic nanoparticles but also improved their efficacy in neutralizing ETX. The study demonstrated that nebulized inhalation of MNPs provides a direct and effective method for delivering treatment against inhaled toxins, a significant advancement over traditional intravenous delivery systems. The long circulation time and targeted delivery provided by RBC-NPs underscore the potential applicability of this approach in treating a variety of toxin-mediated infections.
Conclusion
The successful development of the membrane-camouflaged nanoparticle (MNP) drug delivery system represents a significant advancement in the therapeutic management of Clostridium perfringens ε-toxin (ETX) infections. This achievement was made possible through a systematic evaluation of the safety and efficacy of MNPs, utilizing membranes derived from red blood cells (RBCs) and Madin-Darby canine kidney (MDCK) cells. By leveraging the unique properties of RBC membranes—such as their biocompatibility and low immunogenicity—the study demonstrated that RBC-derived MNPs can effectively neutralize ETX both in vitro and in vivo.
A highlight of the study is the demonstration that RBC-NPs provide superior protection against ETX intoxication compared to MDCK-NPs, with a 100% survival rate observed in treated mice following exposure to the toxin. Furthermore, the innovative delivery methods, including both intravenous injection and nebulized inhalation, proved effective in enhancing the therapeutic potential of the MNPs, showcasing their versatility in addressing different routes of toxin exposure. Overall, this research underscores the promise of MNPs as a novel therapeutic platform for combating toxin-mediated infections, paving the way for future studies and applications in the field of nanomedicine.
Reference:
Xu, Jinglin, et al. “Systematic evaluation of membrane-camouflaged nanoparticles in neutralizing Clostridium perfringens ε-toxin.” Journal of Nanobiotechnology, vol. 21, no. 95, 2023.
