Editor: Tiffany
Researchers have developed Rh-Mito, a small molecule that targets mitochondrial DNA to activate the cGAS-STING pathway, enhancing cancer immunotherapy by triggering immune responses and altering epigenetic regulation.
Key Highlights
- Research Question:
How can a small molecule targeting mitochondrial DNA activate the cGAS-STING pathway to improve cancer immunotherapy? - Research Difficulties:
Overcoming the instability and delivery challenges of existing STING agonists, such as cyclic dinucleotides, and understanding mtDNA’s role in immune activation. - Key Findings:
Rh-Mito intercalates into mtDNA, causing its release into the cytoplasm, which activates the cGAS-STING pathway and enhances antitumor immunity; it also alters mitochondrial metabolites affecting nuclear DNA methylation. - Innovative Aspects:
First small molecule mtDNA intercalator to activate cGAS-STING signaling; use of HFn nanocages for systemic delivery; demonstration of mitochondrial retrograde signaling’s role in immune modulation. - Importance of the Study:
Provides a new strategy for cancer immunotherapy by targeting mtDNA, potentially overcoming limitations of current treatments and offering insights into mitochondrial-immune interactions.
Cancer Immunotherapy and the Role of Mitochondrial DNA
Cancer comprises a range of diseases characterized by uncontrolled cell growth and the ability to spread to other parts of the body. This diversity has prompted the development of new treatment strategies, including immunotherapy. Immunotherapy utilizes the body’s immune system to detect and destroy cancer cells, providing a focused alternative to conventional therapies. A key mechanism in this approach is the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway, which plays an essential role in innate immunity. This pathway senses cytosolic DNA and triggers the production of type I interferons and inflammatory cytokines, activating immune cells such as dendritic cells (DCs) and T cells to target cancer.
Mitochondrial DNA (mtDNA) serves as an important activator of the cGAS-STING pathway. Unlike nuclear DNA, mtDNA is located within mitochondria and can be released into the cytoplasm under conditions of cellular stress, such as damage or metabolic disruption. In the cytoplasm, mtDNA functions as a danger-associated molecular pattern (DAMP), binding to cGAS and initiating STING-mediated immune responses. This process is particularly significant in cancer, where mtDNA release can strengthen antitumor immunity by signaling the presence of malignant cells to the immune system.
Existing immunotherapies targeting the cGAS-STING pathway include cyclic dinucleotides (CDNs), such as cGAMP, and non-nucleotide STING agonists. CDNs bind directly to STING, promoting immune cell activation, but their clinical use is hindered by low metabolic stability and limited systemic bioavailability, often requiring direct injection into tumors. Non-nucleotide STING agonists address some of these challenges but typically need to be combined with other treatments, such as immune checkpoint inhibitors, for meaningful effects. These limitations highlight the need for new agents that can activate the cGAS-STING pathway with improved stability and delivery methods.
Developing a Novel mtDNA-Targeting Agent for Enhanced Immunotherapy
The study aimed to address the limitations of current STING agonists by developing Rh-Mito, a small molecule designed to target mtDNA and enhance cGAS-STING-mediated immunotherapy. Challenges in the field include the rapid breakdown of CDNs due to hydrolysis and a limited understanding of mtDNA’s role in immune activation in cancer. The main goal was to synthesize and test Rh-Mito, a rhodium(III) complex, for its ability to intercalate into mtDNA, cause its damage, and facilitate its release into the cytoplasm to activate the cGAS-STING pathway. Additional goals included assessing Rh-Mito’s effects on mitochondrial metabolism and its downstream impact on nuclear DNA methylation, which could further influence immune responses.
The research was conducted by a team from the MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry, Sun Yat-sen University, including Yue Zheng, Xiao-Xiao Chen, Zong-Wan Mao, Cai-Ping Tan, and collaborators. The results were published on June 5, 2023, in Chemical Science, a peer-reviewed journal dedicated to advancing chemical research.
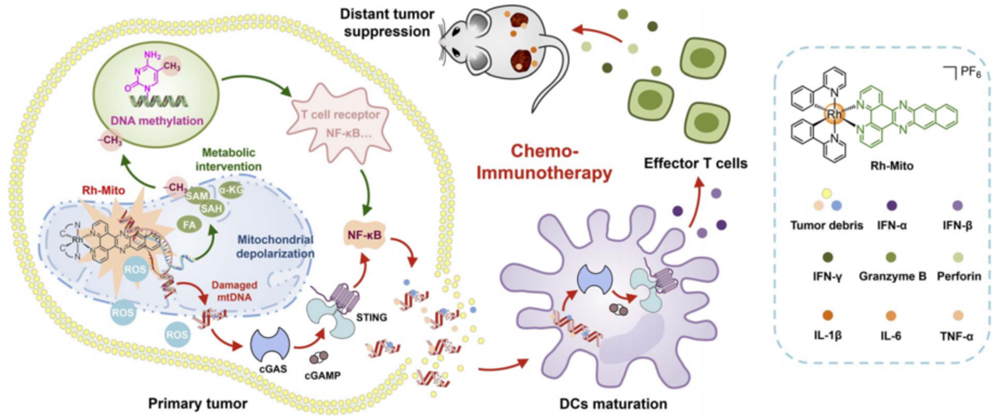
Figure 1. Schematic illustration of the mechanisms of chemo-immunotherapeutic responses evoked by Rh-Mito.
Experimental Validation of Rh-Mito’s Effects on mtDNA and Immune Activation
The study involved the synthesis and characterization of Rh-Mito, in vitro experiments to evaluate its interaction with mtDNA and cellular effects, multi-omics analysis to investigate metabolic and epigenetic changes, and in vivo studies in mouse models to assess therapeutic potential. Below are detailed accounts of four key experiments, including their methods, findings, and contributions.
1. mtDNA Binding In Vitro
- Procedure: Rh-Mito was synthesized, and its DNA-binding affinity was measured using UV/Vis spectroscopy and viscosity tests with calf thymus DNA (CT-DNA) as a model. Its mitochondrial specificity was confirmed through cellular localization studies.
- Result: Rh-Mito showed a binding constant (Kb) of 5.97 × 10^6 M^-1 and increased CT-DNA viscosity, indicating strong intercalation. It primarily localized to mitochondria, with minimal interaction with nuclear DNA or proteins.
- Finding: Rh-Mito has a high affinity for mtDNA, functioning as a selective intercalator that targets mitochondrial DNA over nuclear DNA.
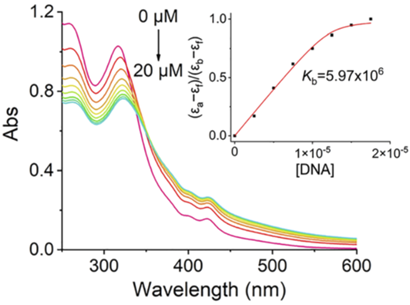
Figure 2. UV/Vis spectra of Rh-Mito (10 mM) titrated with CT-DNA (0–20 mM) in Tris–HCl buffer. The arrow shows the alteration in the absorption intensity of Rh-Mito upon the addition of CT-DNA.
2. mtDNA Damage in HeLa Cells
- Procedure: HeLa cervical cancer cells were treated with Rh-Mito, and mtDNA integrity was analyzed using gel electrophoresis. Reactive oxygen species (ROS) levels, an indicator of mitochondrial stress, were measured with H_2DCFDA staining.
- Result: Rh-Mito treatment decreased mtDNA copy number by 75% and markedly increased mitochondrial ROS levels, suggesting oxidative damage.
- Finding: Rh-Mito causes mtDNA fragmentation and release into the cytoplasm, a key step in triggering cGAS-STING signaling, by disrupting mitochondrial function.
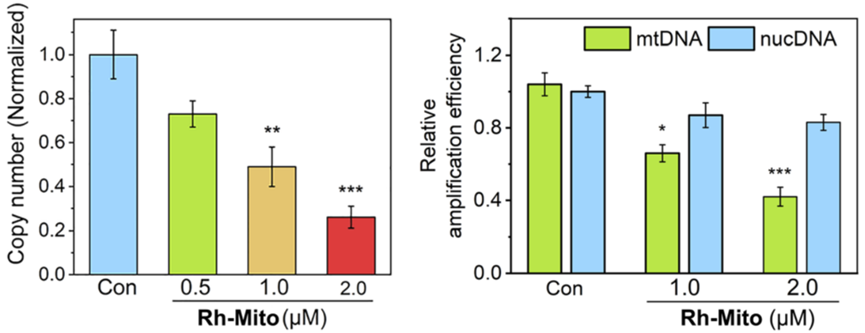
Figure 3. Impact of Rh-Mito on mtDNA copy number and amplification.
3. cGAS-STING Activation in HeLa Cells and DCs
- Procedure: HeLa cells and dendritic cells (DCs) were exposed to Rh-Mito, and cGAS-STING activation was assessed by measuring cGAMP levels with ELISA and detecting phosphorylated STING (p-STING) expression via western blotting.
- Result: Rh-Mito elevated cGAMP production and p-STING levels in both cell types, confirming activation of the pathway.
- Finding: Rh-Mito activates the cGAS-STING pathway by promoting mtDNA release into the cytoplasm, enhancing immune signaling in cancer cells and antigen-presenting cells like DCs.
4. In Vivo Testing with Rh-Mito@HFn Nanoparticles
- Procedure: Rh-Mito was encapsulated in human heavy-chain ferritin (HFn) nanocages to improve systemic delivery and administered intravenously to U14 tumor-bearing mice. Tumor growth, DC maturation, and CD8+ T cell infiltration were tracked.
- Result: Rh-Mito@HFn reduced primary tumor growth by 95.4% and distant tumors by 86.1%, while increasing DC maturation and CD8+ T cell presence in the tumor microenvironment.
- Finding: Encapsulation in HFn nanocages enables Rh-Mito to stimulate systemic antitumor immunity, highlighting its potential as an immunotherapeutic agent with enhanced delivery.
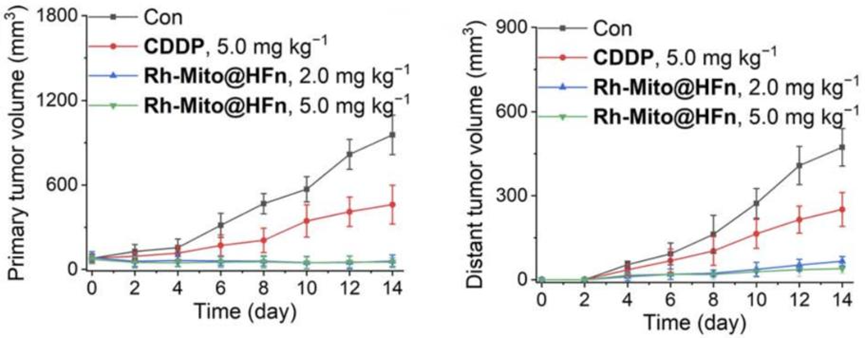
Figure 4. The volume curves of primary tumors and distant tumors upon different treatments.
Rh-Mito as a Potential Immunotherapeutic Agent
This study presents Rh-Mito as a small molecule that targets mtDNA to strengthen cancer immunotherapy through the cGAS-STING pathway. By intercalating into mtDNA, Rh-Mito induces its damage and release into the cytoplasm, activating cGAS-STING signaling and promoting DC maturation and CD8+ T cell infiltration. Additionally, Rh-Mito alters mitochondrial metabolites involved in epigenetic regulation, influencing nuclear DNA methylation and enhancing immune gene expression. Encapsulating Rh-Mito in HFn nanocages improves its bioavailability and antitumor efficacy in vivo, addressing limitations of existing STING agonists like CDNs.
The research provides new insights, including the demonstration of a small molecule mtDNA intercalator activating cGAS-STING signaling and the influence of mitochondrial-to-nuclear communication on immune responses. These findings suggest Rh-Mito as a viable candidate for systemic cancer immunotherapy and enhance our understanding of how mitochondrial dysfunction can be leveraged to improve cancer treatment. The study tackles key challenges in the field and lays the groundwork for future exploration of mtDNA-targeted immunotherapies.
Reference:
Zheng, Yue, et al. “Activation of the cGAS-STING pathway by a mitochondrial DNA-targeted emissive rhodium (iii) metallointercalator.” Chemical Science 14.25 (2023): 6890-6903.
