Editor: Tiffany
Researchers have developed a carbon dots-based nanozyme that co-delivers doxorubicin and siRNA to effectively address drug-resistant lung cancer, potentially enhancing treatment options for affected patients.
Key Highlights
- Research Question:
How can a co-delivery system utilizing carbon dots effectively overcome chemoresistance in lung cancer by targeting the MRP1 protein? - Research Difficulties:
The study addresses the challenges of drug resistance in lung cancer, particularly the efflux of chemotherapeutics due to multidrug resistance proteins like MRP1. - Key Findings:
The carbon dots-based nanozyme effectively silenced the MRP1 gene, leading to increased accumulation of doxorubicin in cancer cells and significant tumor growth inhibition in xenograft models. - Innovative Aspects:
This study combines the delivery of chemotherapeutics and siRNA in a single nanoformulation, utilizing carbon dots that have intrinsic enzyme-like properties to enhance therapeutic efficacy. - Importance of the Study:
The findings suggest a promising new approach to enhance the effectiveness of chemotherapy in lung cancer patients who exhibit drug resistance, potentially improving survival rates.
Context of Lung Cancer Treatment and Chemoresistance
Lung cancer remains the leading cause of cancer-related mortality worldwide, with a particularly high incidence of chemoresistance complicating treatment. Conventional therapies, such as chemotherapy, often fail due to the efflux of drugs from cancer cells, primarily mediated by multidrug resistance proteins, notably the MRP1 protein. This protein, along with others like P-glycoprotein, actively transports chemotherapeutic agents out of the cells, reducing drug efficacy and contributing to poor prognoses for patients. Despite advancements in targeted therapies and immunotherapy, the need for innovative strategies to enhance drug delivery and overcome resistance remains critical. Nanotechnology, particularly through the use of carbon dots, offers a promising avenue for developing effective co-delivery systems that can simultaneously deliver chemotherapeutics and targeted RNA, potentially improving treatment outcomes for lung cancer patients.
Research Aim & Objectives
This study aims to investigate the efficacy of a novel carbon dots-based co-delivery system for overcoming chemoresistance in lung cancer, specifically targeting the MRP1 protein. The primary objectives include: 1) synthesizing and characterizing a carbon dots-polyethyleneimine (CD-PEI) nanoformulation capable of co-delivering doxorubicin (DOX) and small interfering RNA (siRNA) targeting MRP1; 2) evaluating the impact of the CD-PEI-DOX-siMRP1 system on drug accumulation and cytotoxicity in drug-resistant lung cancer cells; and 3) assessing the therapeutic efficacy of this co-delivery system in vivo using xenograft models, with a particular focus on tumor growth inhibition and modulation of MRP1 expression. By achieving these objectives, the study aims to provide a foundation for developing more effective treatment strategies against drug-resistant lung cancer.
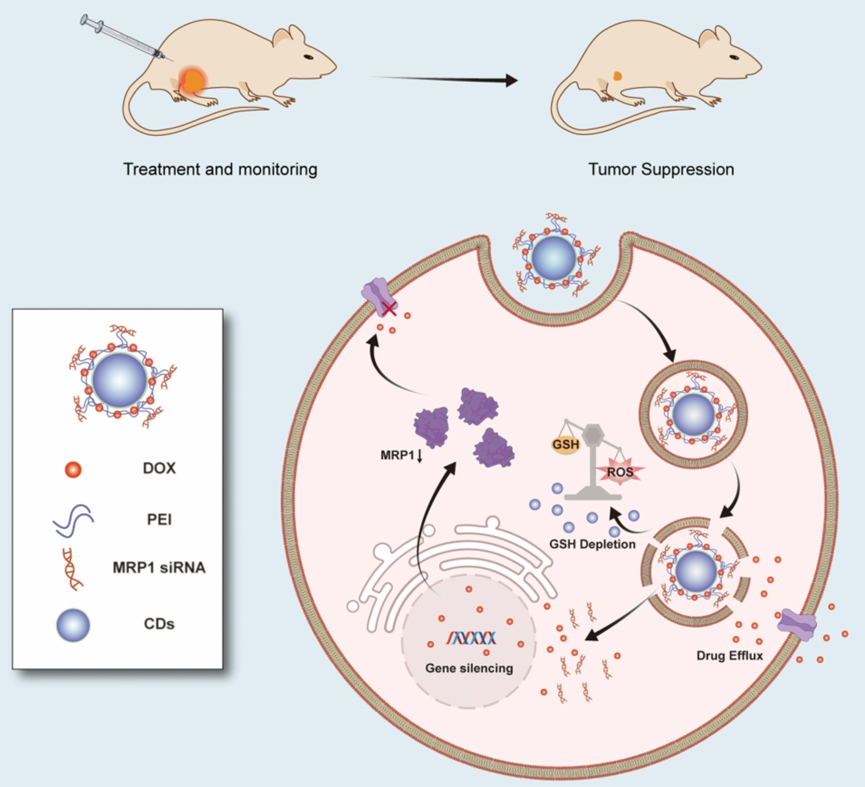
Figure 1. The schematic diagram delineates how CD-PEI-DOX-siMRP1 delivers doxorubicin to tumors and antagonizes chemoresistance by hindering drug efflux through knocking down MRP1 expression.
Experimental Design and Key Findings
(1) Experimental Process Outline
- Synthesis of CD-PEI: Carbon dots were synthesized using a one-step microwave-assisted method and surface-modified with polyethyleneimine (PEI).
- Characterization of CD-PEI: Evaluated using transmission electron microscopy (TEM), UV-Vis spectroscopy, and fluorescence spectroscopy.
- Loading and release of DOX and siRNA: Doxorubicin (DOX) was loaded onto CD-PEI through electrostatic interactions, followed by the addition of siRNA targeting MRP1.
- In vitro cytotoxicity assays: Conducted using A549 and A549/ADM lung cancer cell lines to assess cell viability after treatment with CD-PEI-DOX and CD-PEI-DOX-siMRP1.
- Intracellular drug release studies: Analyzed the release of DOX in cancer cells using confocal microscopy.
- In vivo xenograft studies: Evaluated therapeutic efficacy and biodistribution of CD-PEI-DOX-siMRP1 in tumor-bearing mice.
- Western blot analysis: Assessed expression levels of MRP1 and related proteins in treated cells and tumor tissues.
- Flow cytometry: Analyzed the uptake of DOX and apoptosis in cancer cells after treatment.
(2) Key Experiments Introduction
1. Synthesis and Characterization of CD-PEI
- Procedure: Carbon dots were synthesized through a one-step microwave-assisted method, followed by surface modification with polyethyleneimine (PEI). The resulting CD-PEI was characterized using TEM to determine size and morphology, as well as UV-Vis and fluorescence spectroscopy to assess optical properties.
- Result: The characterization revealed that CD-PEI had an average size of approximately 5.5 nm and exhibited strong fluorescence under UV light, confirming successful synthesis and modification.
- New Finding: The successful modification of carbon dots with PEI enhanced their biocompatibility and stability, providing a promising platform for drug delivery.
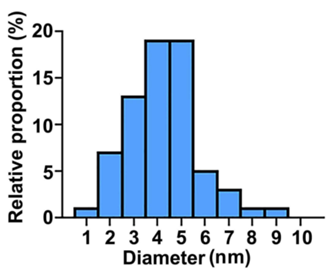
Figure 2. The diameter distribution of CD-PEI.
2. In vitro Cytotoxicity Assays
- Procedure: A549 and A549/ADM cells were treated with various formulations including free DOX, CD-PEI-DOX, and CD-PEI-DOX-siMRP1. Cell viability was measured using the CCK8 assay after 48 hours.
- Result: The IC50 values indicated that CD-PEI-DOX-siMRP1 significantly reduced cell viability in A549/ADM cells compared to free DOX and CD-PEI-DOX alone, demonstrating enhanced cytotoxicity.
- New Finding: The combination of siRNA targeting MRP1 with DOX in the co-delivery system not only increased drug accumulation in resistant cells but also effectively overcame chemoresistance.
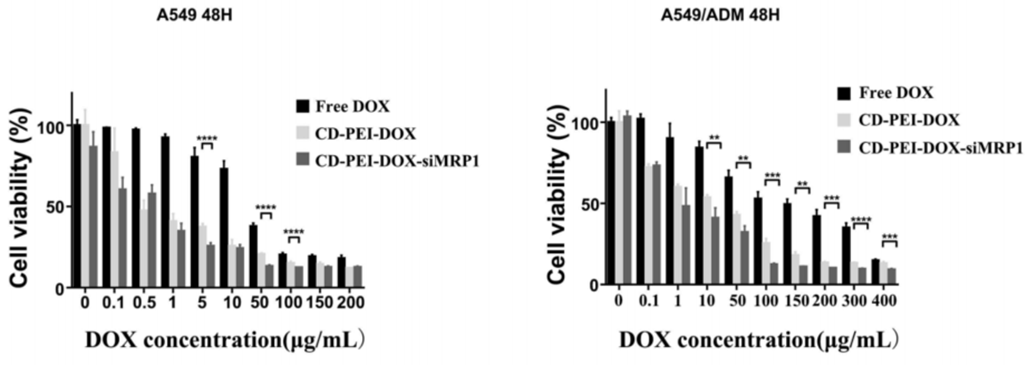
Figure 3. The cell viability of A549 and A549/ADM cells incubated with free DOX, CD-PEI-DOX, and CD-PEI-DOX-siMRP1 for 48 hours were analyzed by CCK8 assays.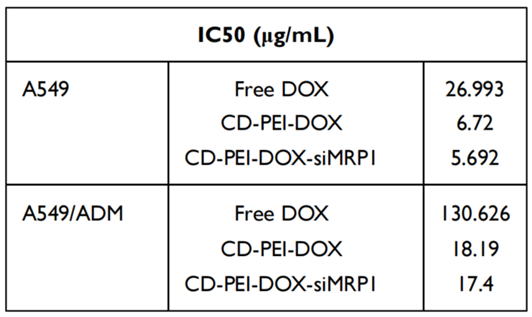
Table 1. IC50 of Free DOX, CD-PEI-DOX, CD-PEI-DOX-siMRP1 in A549 and A549/ADM Cells.
3. In vivo Xenograft Studies
- Procedure: A549/ADM cells were implanted in nude mice to establish xenograft tumors. Mice were treated with various formulations through tail vein injection, followed by monitoring tumor growth and drug biodistribution using in vivo imaging.
- Result: Mice treated with CD-PEI-DOX-siMRP1 displayed reduced tumor volume and weight compared to control groups, indicating effective tumor inhibition.
- New Finding: The co-delivery system showed significant potential in enhancing drug retention in tumor tissues while simultaneously silencing MRP1, thereby improving therapeutic efficacy against chemoresistant lung cancer.
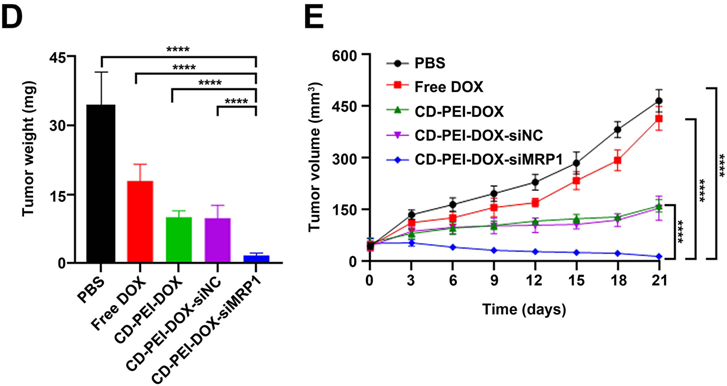
Figure 4. (D) Tumors from each group were weighed and shown in the graph. (E) Tumor volume were recorded and plotted from each group after the indicated treatment.
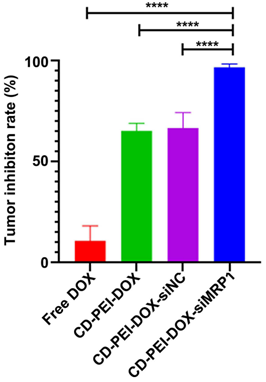
Figure 5. Tumor inhibition rate was calculated from each group.
Implications of Findings for Lung Cancer Therapy
This study presents a novel approach to overcoming chemoresistance in lung cancer through a carbon dots-based co-delivery system that combines doxorubicin (DOX) and small interfering RNA (siRNA) targeting the MRP1 protein. The key findings indicate that the CD-PEI-DOX-siMRP1 formulation significantly enhances intracellular drug accumulation, effectively silencing MRP1 expression, and thereby improving the cytotoxic effects of DOX in resistant lung cancer cells. Importantly, the co-delivery system resulted in tumor growth inhibition in xenograft models, without causing significant toxicity to normal tissues. These findings highlight the potential of using carbon dots as multifunctional carriers to not only deliver chemotherapeutics but also modulate resistance mechanisms within cancer cells. This innovative strategy contributes to advancements in therapeutic options for lung cancer treatment, addressing a critical need for effective therapies against drug-resistant tumors.
Reference:
Yu, Hailing, et al. “Carbon dots-based nanozyme for drug-resistant lung cancer therapy by encapsulated doxorubicin/siRNA cocktail.” International journal of nanomedicine (2023): 933-948.
