Editor: Nina
Researchers create a capecitabine-loaded prebiotic nanoparticle that enhances colorectal cancer treatment through gut microbiota modulation and improved drug delivery, leading to increased therapeutic efficacy and reduced side effects.
Key Preview
Research Question
The study investigates whether combining gut microbiota modulation through a prebiotic nanoparticle with chemotherapy using capecitabine can enhance the therapeutic efficacy against colorectal cancer (CRC).
Research Design and Strategy
The researchers developed a capecitabine-loaded prebiotic nanoparticle, termed SCXN, utilizing a xylan-stearic acid conjugate. This nanoparticle was designed for oral administration to improve drug delivery and therapeutic outcomes in CRC.
Method
The efficacy of SCXN was evaluated in CRC mouse models, comparing it to free capecitabine. Key metrics included intra-tumoral drug concentration, tumor growth inhibition, and overall survival rates.
Key Results
SCXN significantly enhanced intra-tumoral capecitabine concentration, raised the tumor inhibition rate from 5.29% to 71.78%, and increased median survival time from 14 days to 33.5 days in treated mice.
Significance of the Research
This research presents a promising strategy for improving CRC therapy by integrating gut microbiota modulation with chemotherapy, potentially leading to enhanced patient outcomes and reduced side effects.
Introduction
Colorectal cancer (CRC) is one of the most prevalent malignancies globally and a leading cause of cancer-related mortality, with significant morbidity affecting individuals across various age groups. The disease is characterized by the uncontrolled proliferation of abnormal cells in the colon or rectum, often resulting in symptoms such as changes in bowel habits, rectal bleeding, and unexplained weight loss. Early detection and intervention are crucial for improving patient outcomes; however, many patients are diagnosed at advanced stages, complicating treatment.
In traditional CRC treatment, systemic chemotherapy remains a cornerstone therapy, with capecitabine being one of the first-line agents. The conventional strategy for drug delivery often relies on intravenous administration, which aims to achieve high drug concentrations in the tumor while minimizing exposure to healthy tissues. However, this approach presents distinct challenges, including the rapid clearance of the drug from circulation, resulting in suboptimal therapeutic concentrations at the tumor site and increased risk of systemic side effects. Additionally, the lack of targeted delivery can lead to diminished effectiveness and the necessity for higher doses, further exacerbating toxicity.
To address these challenges, innovative drug delivery strategies are being explored. One promising approach involves the development of nanoparticles that encapsulate chemotherapeutic agents while simultaneously modulating the gut microbiota. This method aims to enhance the localized delivery of drugs directly to the tumor site, prolonging circulation time and improving therapeutic efficacy while potentially mitigating adverse effects associated with traditional chemotherapy. The current study focuses on creating a capecitabine-loaded prebiotic nanoparticle, designed to optimize drug delivery and leverage the benefits of gut microbiota modulation to improve outcomes in CRC treatment.
Research Team and Aim
The research was conducted by a team from the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, led by Tianqun Lang. The study was carried out over the past few years, culminating in the publication of the paper titled “Combining gut microbiota modulation and chemotherapy by capecitabine-loaded prebiotic nanoparticle improves colorectal cancer therapy” in the journal Nature Communications.
The aim of the research, as articulated by lead researcher Tianqun Lang, was to explore the synergistic effects of prebiotic nanoparticles on the efficacy of capecitabine in treating colorectal cancer (CRC), specifically to enhance drug delivery, improve tumor targeting, and regulate gut microbiota to foster a more favorable immune response against tumors.
Experimental Process
Primary Technique
The primary technique employed in this study was the development and evaluation of a capecitabine-loaded prebiotic nanoparticle, termed SCXN. This technique combined the advantages of nanoparticle drug delivery systems with gut microbiota modulation to enhance the therapeutic efficacy of capecitabine against colorectal cancer (CRC).
1. Nanoparticle Synthesis
Key Steps:
- The synthesis of SCXN involved the conjugation of xylan, stearic acid (Sa), and capecitabine (Cap).
- Xylan (2.00 g), N,N’-dicyclohexylcarbodiimide (DCC, 6.24 g), and 4-(dimethylamino)-pyridine (DMAP, 1.76 g) were dissolved in dimethylsulfoxide (DMSO, 80 mL).
- A solution of Sa (4.31 g in DMSO, 40 mL) was added dropwise to the mixture, and the reaction was stirred at room temperature for 24 hours.
- The resultant solution was precipitated in 95% ethanol (2 L), and the precipitate was collected via centrifugation (6000 g, 5 min) at 4 °C.
Data Collection and Analysis:
- The structures of the synthesized compounds (Sxy and Scap) were confirmed using 1H-nuclear magnetic resonance (NMR) spectroscopy and matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS).
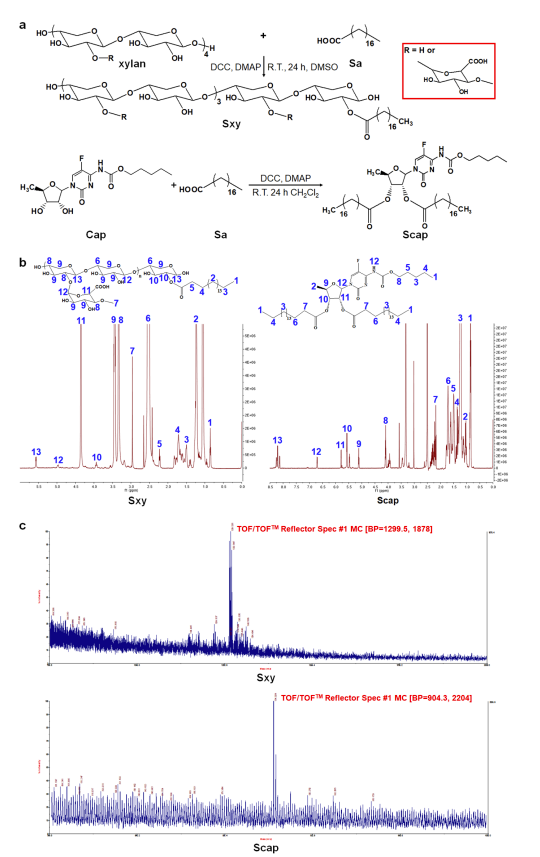
Figure 1. Synthesis and structure confirmation of Sxy and Scap. a, The diagram of the synthesis of Sxy and Scap. b, The 1H NMR spectra of Sxy and Scap. c, The MALDI-TOF-MS spectra of Sxy and Scap.
Novel Aspects:
- This method established an efficient way to create amphiphilic derivatives that could facilitate the encapsulation of Cap, enhancing its solubility and stability, which are crucial for oral delivery.
2. In Vivo Evaluation
Key Steps:
- CRC mouse models (CT26) were developed by intraperitoneally injecting 1 × 10^6 CT26 cells into Balb/c mice.
- Mice were randomly assigned into treatment groups that received either free capecitabine or SCXN via oral administration at a dose of 50 mg/kg Cap.
Data Collection and Analysis:
- Tumor growth was monitored using bioluminescence imaging (IVIS) and tumor volumes were calculated based on photon emission over time.
- Statistical analysis was performed using a two-sided log-rank (Mantel–Cox) test for survival curves and one-way ANOVA for tumor volume comparisons.

Figure 2. In vivo anti-tumor effects in CT26-luc tumor-bearing mice receiving multi-dose treatments of different formulations.
Novel Aspects:
- The use of fluorescence imaging allowed for real-time monitoring of drug accumulation in tumors, a significant advancement over traditional methods that often rely on post-mortem analysis.
3. Biodistribution Studies
Key Steps:
- The biodistribution of the SCXN was evaluated using Sxy-DiR nanoparticles, which were administered to CT26-luc tumor-bearing mice.
- Mice were imaged using IVIS at various time points (1, 4, 8, and 24 hours) post-administration to assess the distribution of the fluorescent dye.
Data Collection and Analysis:
- Ex vivo fluorescence imaging of major organs (heart, liver, spleen, lungs, kidneys, and tumors) was conducted to quantify the accumulation of DiR at different time intervals.
- Quantitative data on drug concentrations in tumor tissues were collected and analyzed using HPLC.
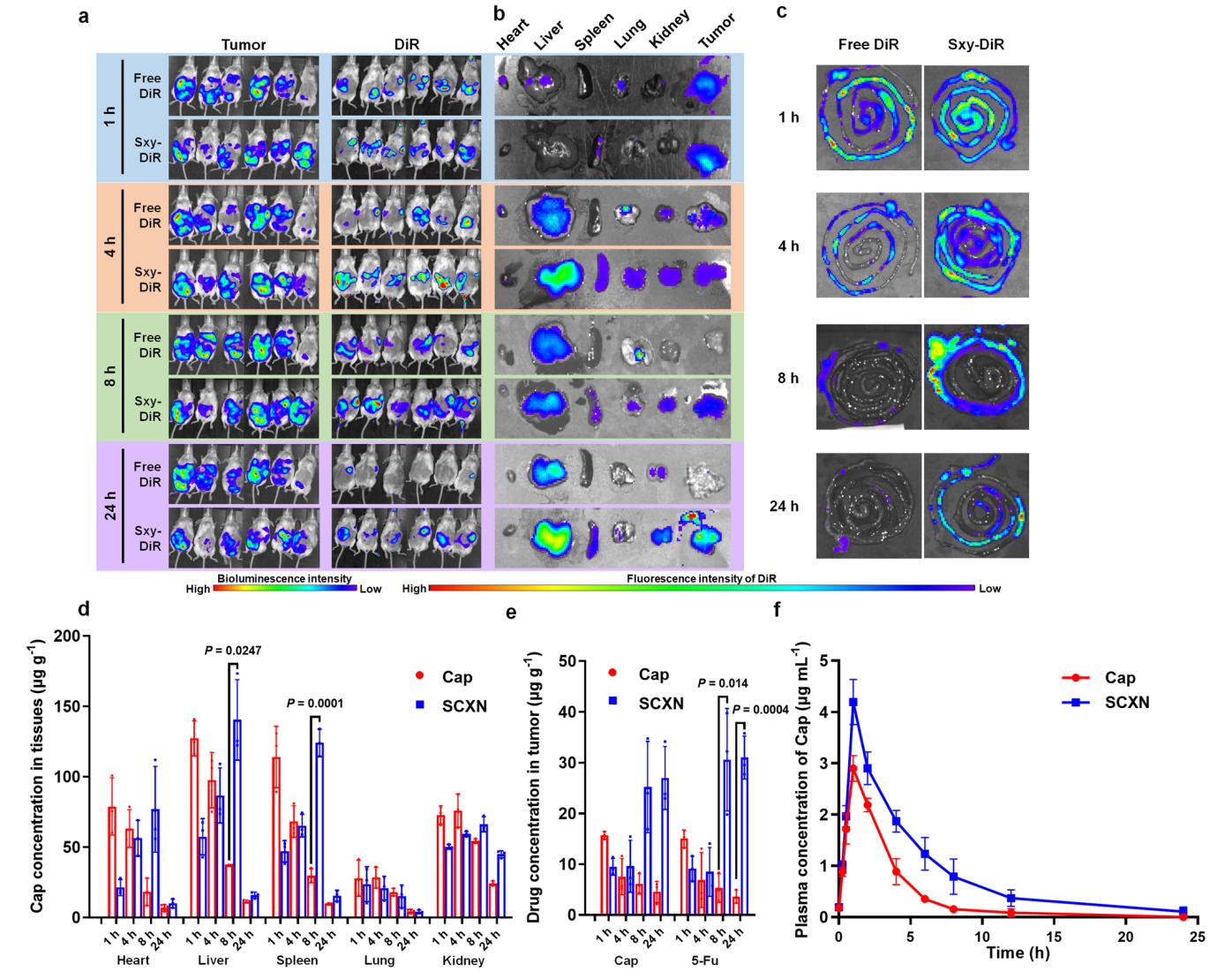
Figure 3. Biodistribution and pharmacokinetics of SCXN.
Novel Aspects:
- The study introduced a novel fluorescent tagging technique that allowed for the visualization and quantification of nanoparticle accumulation in vivo, providing insights into pharmacokinetics that traditional assays could not achieve.
4. Immunological Assessment
Key Steps:
- The immune response to SCXN treatment was assessed by analyzing the populations of dendritic cells (DCs) and CD8+ T cells in draining lymph nodes and tumors.
- Tumor tissues were harvested, and single-cell suspensions were prepared for flow cytometry analysis.
Data Collection and Analysis:
- Cells were stained with fluorescent-conjugated antibodies and analyzed by flow cytometry to quantify populations of immune cells, such as matured DCs and cytotoxic T lymphocytes.
- Statistical significance was assessed using one-way ANOVA.
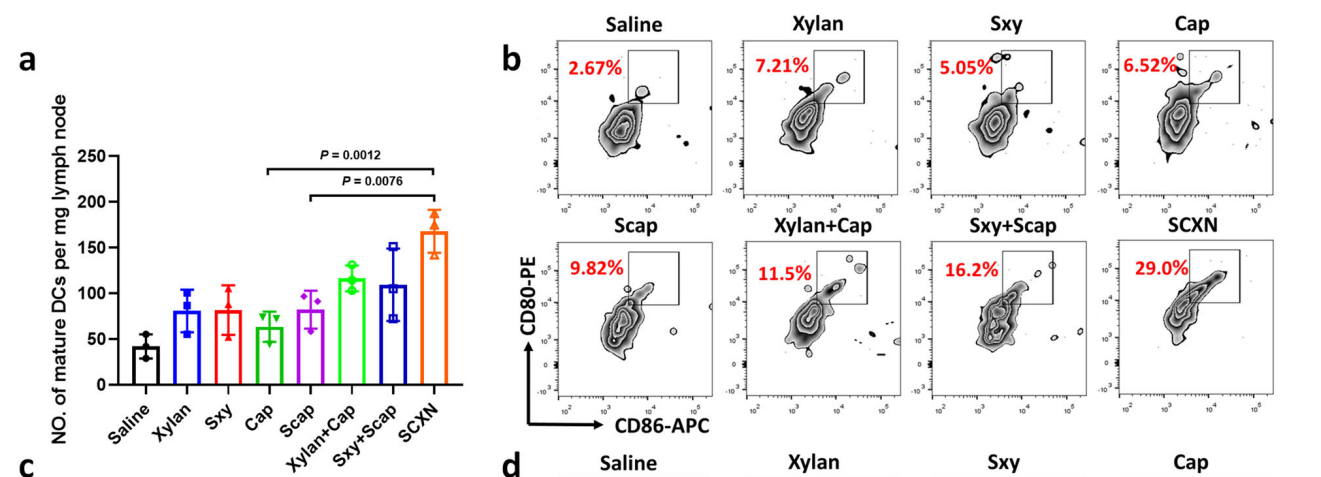
Figure 4. a, b Numbers (a) and percentages (b) of mature dendritic cells (DCs; CD80+ CD86+ cells gated on CD11c+ cells) in draining lymph nodes.
Novel Aspects:
- This approach provided a comprehensive analysis of the immune landscape in response to SCXN treatment, highlighting the dual role of prebiotic nanoparticles in enhancing both drug delivery and immune modulation compared to conventional chemotherapy alone.
5. Gut Microbiota Analysis
Key Steps:
- Fecal samples were collected from treated mice at various time points during the study for microbiota profiling.
- DNA was extracted from fecal samples, and the V3-V4 region of the 16S rRNA gene was amplified for sequencing.
Data Collection and Analysis:
- Sequencing data were analyzed using the Qiime2 platform to identify microbial diversity and community structure.
- Statistical analyses, including non-metric multidimensional scaling (NMDS) and linear discriminant analysis effect size (LEfSe), were performed to assess differences in microbiota composition among treatment groups.
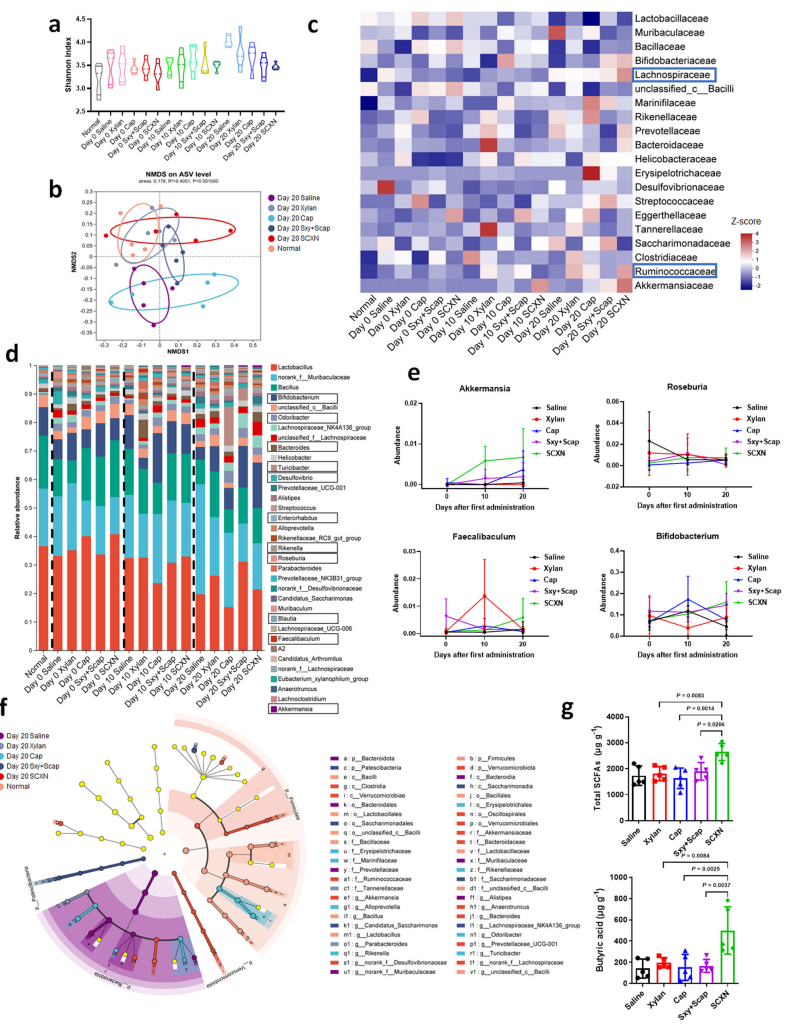
Figure 5. Regulating gut microbiota by SCXN. CT-26 tumor-bearing mice were treated with different formulations for 20 d and fecal samples were collected for 16 S rDNA sequencing before (Day 0), in the middle of (Day 10), and at the end of (Day 20) the treatment.
Novel Aspects:
- This study employed advanced sequencing techniques to thoroughly characterize gut microbiota changes, establishing a link between microbiome modulation and improved therapeutic outcomes that is not typically explored in traditional cancer therapies.
These experiments collectively underscore the innovative approach of utilizing SCXN for CRC treatment by integrating nanotechnology with microbiome science, significantly enhancing both therapeutic efficacy and safety.
Conclusion
The study concluded that the SCXN nanoparticle significantly improves the therapeutic efficacy of capecitabine against CRC by enhancing drug delivery and modulating gut microbiota. The findings suggest that this dual approach may lead to better clinical outcomes compared to traditional chemotherapy alone, although the authors acknowledged limitations, such as the need for further studies to translate these findings into clinical practice. Future research should explore the scalability of this delivery system and its efficacy across diverse cancer types, potentially paving the way for new treatment paradigms in oncology.
This innovative research underscores the potential of integrating nanotechnology and microbiome science to advance cancer therapy, offering hope for improved treatment strategies that harness the body’s own biological mechanisms to combat disease.
Reference
Lang, Tianqun, et al. “Combining gut microbiota modulation and chemotherapy by capecitabine-loaded prebiotic nanoparticle improves colorectal cancer therapy.” Nature Communications, vol. 14, no. 4746, 2023.
