Editor: Nina
Scientists reveal that fatostatin induces ferroptosis in glioblastoma cells by inhibiting the AKT/mTORC1/GPX4 signaling pathway and develop a p28-functionalized PLGA nanoparticle delivery system to enhance targeted treatment efficacy.
Key Preview
Research Question
This study investigates how fatostatin can induce ferroptosis—a form of regulated cell death—by inhibiting the AKT/mTORC1/GPX4 signaling pathway in glioblastoma multiforme (GBM), which is notorious for its aggressive nature and poor prognosis.
Research Design and Strategy
The research employed a combination of transcriptome sequencing, in vivo experiments, and in vitro experiments to analyze the effects of fatostatin on GBM cells and investigate the underlying mechanisms of its action.
Method
The study utilized human GBM cell lines U87 and U251 to assess the impact of fatostatin on cell viability, proliferation, and migration. Various assays, including CCK-8, colony-forming, and Western blot analysis, were employed to elucidate the effects of fatostatin on these cells.
Key Results
Fatostatin was found to inhibit cell proliferation and induce ferroptosis in GBM cells by decreasing GPX4 protein levels through suppression of the AKT/mTORC1 signaling pathway. Quantitative data revealed that the IC50 for U87 cells was 21.38 μM and for U251 cells was 19.44 μM. Additionally, the study demonstrated that fatostatin-loaded PLGA nanoparticles significantly enhanced drug delivery across the blood-brain barrier (BBB).
Significance of the Research
This research highlights a novel therapeutic potential for fatostatin in treating GBM through targeted delivery methods, offering fresh hope in a field where effective treatments are limited.
Understanding Glioblastoma and Drug Delivery Challenges
Glioblastoma multiforme (GBM) is recognized as the most prevalent and aggressive primary malignant brain tumor in adults, accounting for a significant proportion of all gliomas. Characterized by its rapid growth and invasive nature, GBM presents substantial challenges in treatment and management. Despite aggressive therapeutic strategies that include surgical resection, chemotherapy (such as temozolomide), and radiation therapy, the median survival rate for GBM patients remains disappointingly low, typically around 14.6 months. The inherent heterogeneity of the tumor and its ability to infiltrate surrounding brain tissue contribute to therapeutic resistance and recurrence.
Traditionally, drug delivery strategies for GBM largely rely on systemic administration, which often results in suboptimal therapeutic concentrations reaching the tumor site due to the presence of the blood-brain barrier (BBB). This biological barrier effectively restricts the passage of many therapeutic agents, limiting their efficacy and leading to treatment failure. Moreover, conventional approaches frequently cause dose-limiting side effects, as these drugs may affect healthy brain tissue and other organs.
The challenges associated with current drug delivery methods result in inadequate tumor targeting, ineffective treatment, and a high likelihood of tumor recurrence. These issues underscore the urgent need for innovative strategies to enhance drug delivery specifically to GBM while minimizing systemic toxicity.
In light of these challenges, researchers are exploring novel drug delivery systems, such as p28-functionalized PLGA nanoparticles. This innovative approach aims to improve the penetration of therapeutic agents across the BBB and deliver them more directly to the tumor site. By utilizing nanoparticle technology, drugs can be encapsulated, allowing for targeted delivery and controlled release, which may enhance therapeutic efficacy and reduce side effects. This study investigates the potential of fatostatin delivered via these nanoparticles to combat GBM effectively, representing a promising avenue for improving treatment outcomes in this formidable disease.
The Research Team and Their Objectives
The research team comprised a collaborative group of scientists led by Jiayang Cai, along with co-authors Zhang Ye, Yuanyuan Hu, and others, all affiliated with the Renmin Hospital of Wuhan University. This study was conducted in 2023 and was published in the journal Cell Death and Disease under the title “Fatostatin induces ferroptosis through inhibition of the AKT/mTORC1/GPX4 signaling pathway in glioblastoma.”
The primary aim of the research, as articulated by lead researcher Jiayang Cai, was to delineate the mechanisms by which fatostatin induces ferroptosis in glioblastoma multiforme (GBM) and to develop a targeted drug delivery system to enhance therapeutic efficacy against this aggressive cancer.
Experimental Process: A Comprehensive Overview
Primary Technique: Cell Viability Assessment using CCK-8 Assay
The primary technique employed in this study was the Cell Counting Kit-8 (CCK-8) assay, which is widely used to evaluate cell viability and proliferation. This method is based on the colorimetric detection of the formazan dye produced by viable cells, allowing quantitative measurement of cell viability.
Key Steps:
- Cell Seeding: Human GBM cell lines (U87 and U251) were seeded at a density of 5,000 cells per well in 96-well plates.
- Drug Treatment: Cells were treated with varying concentrations of fatostatin and incubated for 24 hours.
- Addition of CCK-8 Reagent: After treatment, 10 µl of CCK-8 solution was added to each well, followed by a 1-hour incubation at 37 °C to allow for the formation of the formazan dye.
- Absorbance Measurement: The absorbance at 450 nm was measured using a Multimode Plate Reader to determine cell viability.
Data Collection and Analysis: The absorbance values were recorded and analyzed to calculate the IC50 values for U87 (21.38 µM) and U251 (19.44 µM) cells. Statistical analysis was performed using GraphPad Prism software, allowing for comparison between control and treated groups.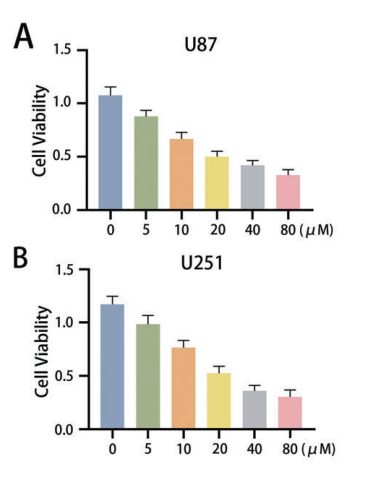
Figure 1. A, B CCK-8 assays were used to measure the cell viability (absorbance value at 450 nm) of U87 and U251 cells after treatment with fatostatin. The IC50 of U87 cells was 21.38 μM, and the IC50 of U251 cells was 19.44 μM.
Novel Aspects: This study utilized the CCK-8 assay not only for initial viability screening but also to derive specific IC50 values, providing a quantitative basis for assessing drug efficacy. The high sensitivity of the CCK-8 assay represents an advantage over traditional methods like trypan blue exclusion, which can be less accurate.
Secondary Technique: Colony Formation Assay
Another critical experimental approach was the colony formation assay, which assessed the long-term effects of fatostatin on cell proliferation and clonogenicity.
Key Steps:
- Cell Preparation: U87 and U251 cells were seeded at a density of 500 cells per well in 6-well plates.
- Treatment with Fatostatin: Cells were treated with various concentrations of fatostatin and incubated for approximately two weeks to allow colony formation.
- Fixation and Staining: After the incubation period, colonies were fixed using 4% paraformaldehyde and stained with 0.1% crystal violet.
- Colony Counting: Visible colonies were photographed and quantified using ImageJ software.
Data Collection and Analysis: The number of colonies formed was counted and compared across different treatment groups to evaluate the impact of fatostatin on clonogenic potential.
Novel Aspects: The use of the colony formation assay provided insights into the long-term effects of fatostatin, highlighting its potential to inhibit tumor growth over time. This assay is more reflective of the in vivo tumor environment compared to short-term viability assays.
Tertiary Technique: Transcriptome Sequencing
Transcriptome sequencing was performed to explore the molecular mechanisms underpinning the effects of fatostatin on GBM cells.
Key Steps:
- RNA Extraction: Total RNA was extracted from U87 cells treated with DMSO (control) and fatostatin using TRIzol reagent.
- Library Preparation: RNA quality was assessed, and cDNA libraries were prepared for sequencing using a NovaSeq 6000 instrument.
- Sequencing and Analysis: Sequencing was performed, and the resulting data were analyzed for differentially expressed genes (DEGs) using the DESeq R package, focusing on genes associated with ferroptosis and the AKT/mTOR pathway.
Data Collection and Analysis: DEGs were determined based on a threshold of |log2(FoldChange)| > 1 and adjusted P value < 0.05. Functional enrichment analyses, including GO and KEGG pathways, were performed to assess biological relevance.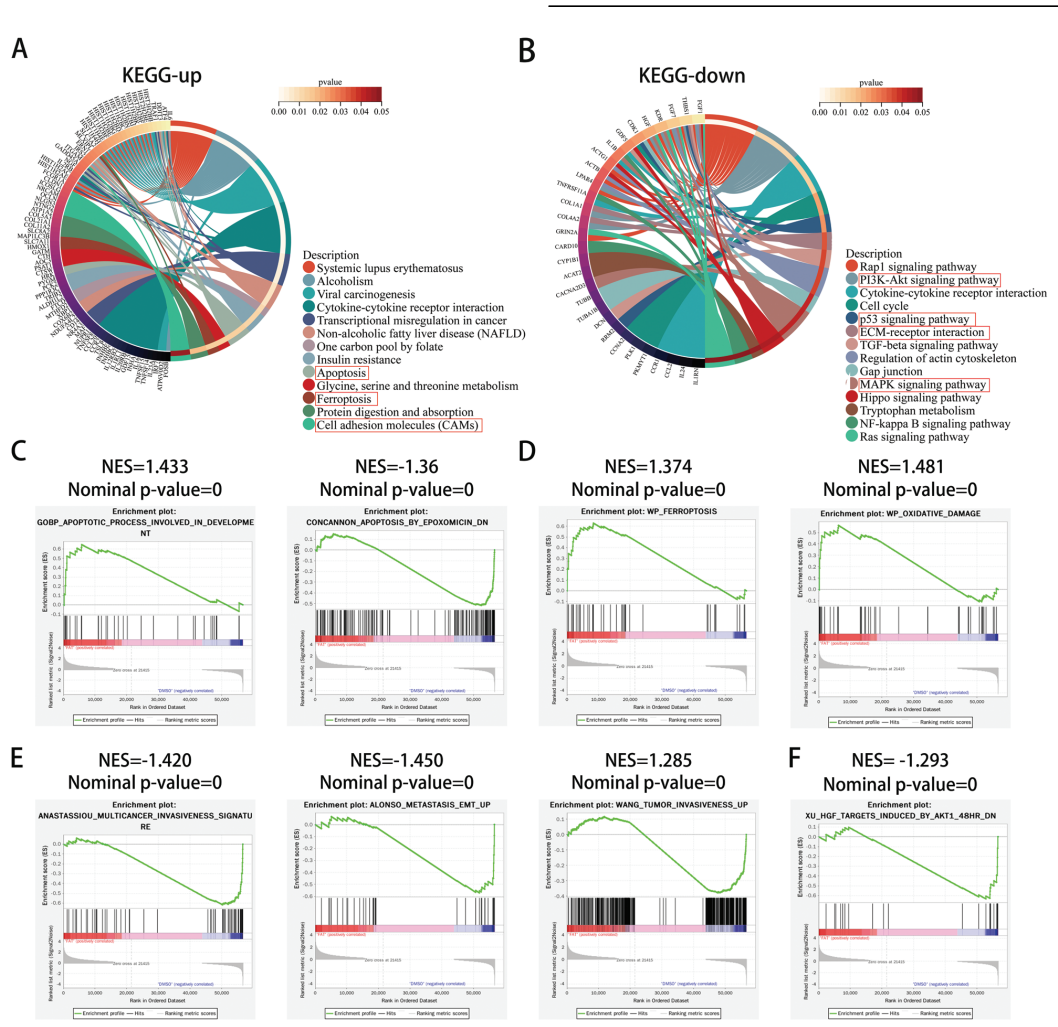
Figure 2. Functional enrichment analysis of DEGs obtained from RNA-seq.
Novel Aspects: The integration of transcriptome sequencing with functional enrichment analysis allowed for a comprehensive understanding of gene expression changes, identifying novel pathways involved in fatostatin-induced ferroptosis. This high-throughput approach offers a broader perspective compared to traditional single-gene analyses.
Quaternary Technique: In Vivo Studies with Nanoparticle Delivery System
To enhance drug delivery to GBM, the study developed p28-functionalized PLGA nanoparticles (NPs) loaded with fatostatin.
Key Steps:
- Nanoparticle Preparation: PLGA and PLGA-PEG-MAL were dissolved in a mixture of methanol and DCM to create an oil phase, which was emulsified in a PVA solution to generate nanoparticles.
- Surface Modification: The peptide p28 was conjugated to the surface of the nanoparticles using maleimidethiol click chemistry.
- Characterization: The size and zeta potential of the nanoparticles were measured using Dynamic Light Scattering (DLS) to confirm successful synthesis.
- In Vivo Administration: The nanoparticles were administered to tumor-bearing mice, and bioluminescence imaging was conducted to monitor tumor progression.
Data Collection and Analysis: The pharmacokinetics of the nanoparticles were assessed through blood sampling at predetermined time points, and tumor growth was analyzed using IVIS imaging.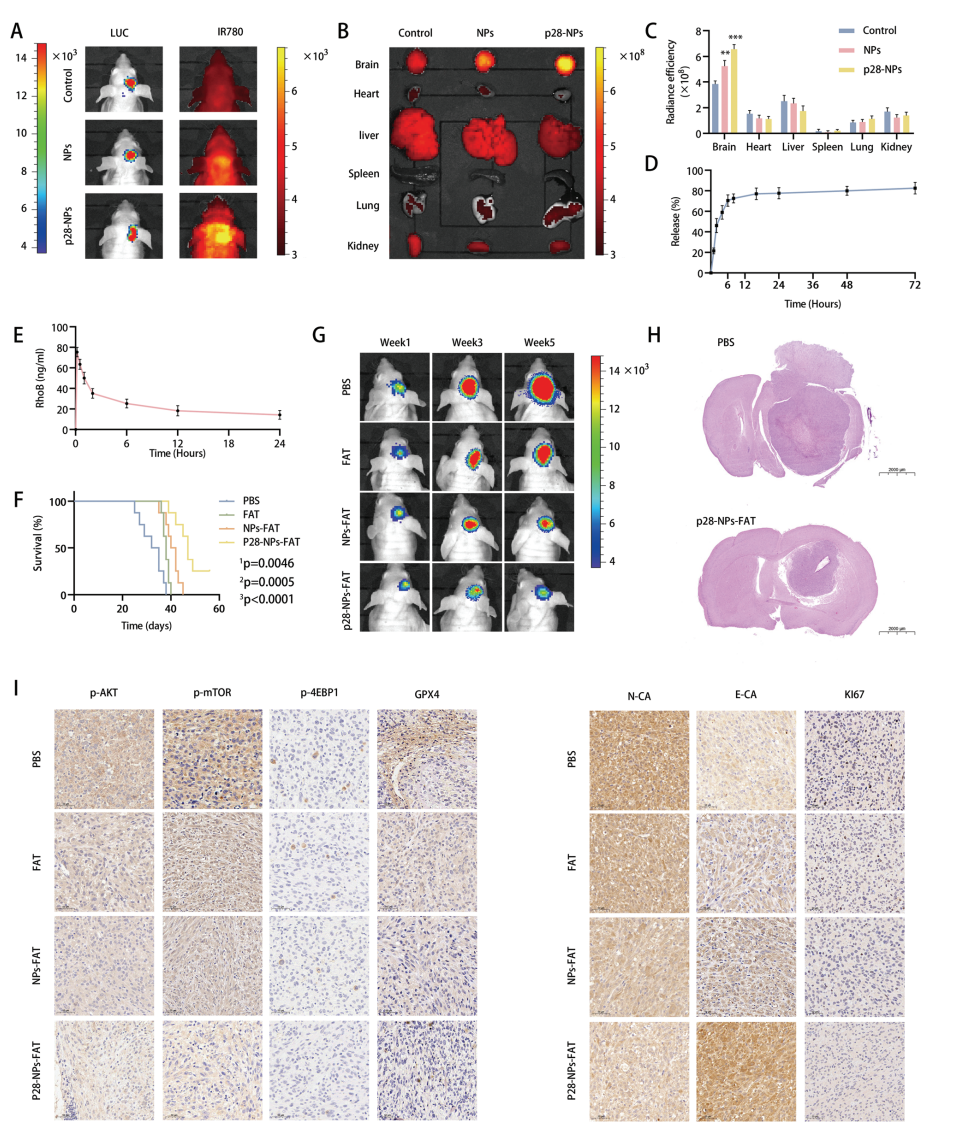
Figure 3. Fatostatin NPs target GBM and inhibit GBM growth in an intracranial xenograft model.
Novel Aspects: The use of p28-functionalized nanoparticles represents a significant advancement in drug delivery systems, allowing for targeted delivery across the blood-brain barrier (BBB) and increasing bioavailability of fatostatin. This approach surpasses traditional delivery methods that often fail to penetrate brain tumors effectively.
In summary, the research was conducted through a comprehensive set of experiments designed to evaluate the effects of fatostatin on GBM cells and enhance its delivery via novel nanoparticle systems. Each technique employed provided critical insights into the efficacy and mechanisms of action of fatostatin, supporting the study’s overarching goal of improving therapeutic strategies for GBM.
Final Thoughts on Research Findings
The successful development of the p28-functionalized PLGA nanoparticle delivery system for fatostatin was achieved through a series of innovative steps that enhanced the drug’s ability to penetrate the blood-brain barrier and target glioblastoma cells effectively. By integrating the p28 peptide with PLGA nanoparticles, the research team was able to create a targeted delivery vehicle that improved the bioavailability of fatostatin, ensuring that higher concentrations of the drug reached the tumor site while minimizing systemic side effects.
The highlights of the study include the demonstration that fatostatin induces ferroptosis in GBM cells by inhibiting the AKT/mTORC1/GPX4 signaling pathway, which represents a novel mechanism of action for this compound in the context of glioblastoma treatment. Additionally, the study established the potential of the p28-functionalized nanoparticles to enhance therapeutic efficacy, providing a promising new strategy for combating the aggressive nature of GBM and improving treatment outcomes for patients affected by this challenging disease.
Reference
Cai, Jiayang, et al. “Fatostatin induces ferroptosis through inhibition of the AKT/mTORC1/GPX4 signaling pathway in glioblastoma.” Cell death & disease 14.3 (2023): 211.
