Editor: Tiffany
Researchers have developed a novel hydrogel microsphere vaccine that significantly boosts immune responses against pancreatic cancer following ablation therapy, potentially improving patient survival rates.
Key Highlights
- Research Question:
Can a hydrogel microsphere vaccine enhance anti-tumor immunity after irreversible electroporation (IRE) ablation therapy for pancreatic cancer? - Research Difficulties:
The main challenge was to overcome the immunosuppressive tumor microenvironment (TME) typical of pancreatic cancer that leads to rapid tumor recurrence after treatment. - Key Findings:
The hydrogel microsphere vaccine effectively transformed the tumor microenvironment from “cold” to “hot,” leading to increased infiltration of CD8+ T cells and improved survival rates in mouse models. - Innovative Aspects:
This study presents the first hydrogel microsphere vaccine designed to amplify endogenous adaptive immune responses through targeted cytokine release specifically in the acidic TME of tumors. - Importance of the Study:
The findings suggest a promising new strategy for enhancing the effectiveness of current treatments for pancreatic cancer, a disease with a very low survival rate.
Pancreatic Cancer Challenges and Immune Landscape
Pancreatic ductal adenocarcinoma (PDAC) is a highly lethal form of cancer, characterized by a 5-year survival rate of less than 7%. The majority of patients are diagnosed at an advanced stage, at which point surgical resection of the tumor is no longer a viable option. Current treatment strategies for PDAC include chemotherapy and minimally invasive ablation therapies, such as irreversible electroporation (IRE), which employs electric fields to destroy cancer cells. Despite these interventions, tumor recurrence remains a significant challenge. This recurrence is largely attributed to the immunosuppressive tumor microenvironment in PDAC, which inhibits the immune system’s ability to target and eliminate cancer cells effectively. Within this microenvironment, type 1 conventional dendritic cells (cDC1) are critical immune cells that can initiate anti-tumor responses by activating T cells, but their function is often suppressed in pancreatic cancer.
Enhancing Immune Responses Through Hydrogel Microsphere Vaccination
The study sought to address the issue of tumor recurrence following ablation therapy by improving the immune system’s capacity to combat pancreatic cancer. The researchers specifically targeted the activation of cDC1 cells, which are essential for triggering anti-tumor immune responses but are hindered by the immunosuppressive conditions within PDAC tumors. To achieve this, they developed a hydrogel microsphere vaccine intended to enhance cDC1 cell activity and thereby strengthen anti-tumor immunity. The research was conducted by a team led by Xiaoyu Liu, Yaping Zhuang, and Wei Huang from Shanghai Jiao Tong University School of Medicine, in collaboration with other institutions, and the findings were published in Nature Communications in 2023.
Innovative Vaccine Design and Efficacy Evaluation
1. Experimental Process Overview
The study aimed to enhance anti-cancer immunity in pancreatic cancer patients through a novel hydrogel microsphere vaccine following irreversible electroporation (IRE) ablation therapy. The experimental process included the following key steps:
- Hydrogel Microsphere Vaccine Development: The vaccine was formulated by loading FLT3L and CD40L into hydrogel microspheres designed for controlled release in the tumor microenvironment.
- Animal Model Establishment: An immunocompetent orthotopic pancreatic cancer mouse model was created using KPC and Panc02 cancer cell lines.
- IRE Ablation Treatment: Mice received IRE treatment to induce tumor necrosis and create a favorable environment for immune activation.
- Vaccine Administration: The hydrogel microsphere vaccine was injected locally into the tumor post-IRE.
- Assessment of Immune Response: Various assays, including flow cytometry, immunohistochemistry, and ELISA, were performed to evaluate immune cell populations and their activity.
- Survival Analysis: The overall survival rates of treated mice were analyzed and compared to control groups.
2. Key Experiments
1. Hydrogel Microsphere Vaccine Preparation
- Procedure:
- The hydrogel microsphere vaccine was synthesized by combining Cas9 plasmid-loaded liposomes and CD40L-loaded CaCO3 nanoparticles with FLT3L and hyaluronic acid under microfluidic conditions.
- The vaccine was designed for dual cytokine release in response to the acidic tumor microenvironment.
- Result:
- The vaccine achieved a cumulative release rate of 48.5% for FLT3L and 54.1% for CD40L over 72 hours at physiological pH.
- New Finding:
- The controlled release of cytokines effectively stimulates the recruitment and activation of type-I conventional dendritic cells (cDC1) in the tumor microenvironment.
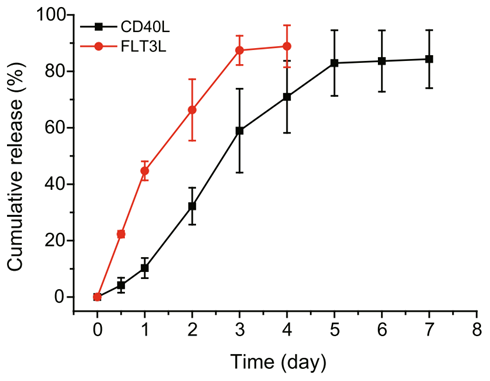
Figure 1. The release of FLT3L and CD40L by the hydrogel microsphere vaccine in vitro (pH = 6.8).
2. Efficacy of the Hydrogel Microsphere Vaccine Combined with IRE
- Procedure:
- After establishing orthotopic tumors, mice underwent IRE ablation, followed by local injection of the hydrogel vaccine.
- Tumor progression and immune cell infiltration were monitored through imaging and histological analysis.
- Result:
- Mice treated with the vaccine showed significant tumor necrosis and a marked increase in the density of cDC1 and CD8+ T cells in the tumor tissue.
- New Finding:
- The combination therapy transformed the tumor microenvironment from “cold” to “hot,” leading to enhanced anti-tumor immunity and significantly improved survival rates.
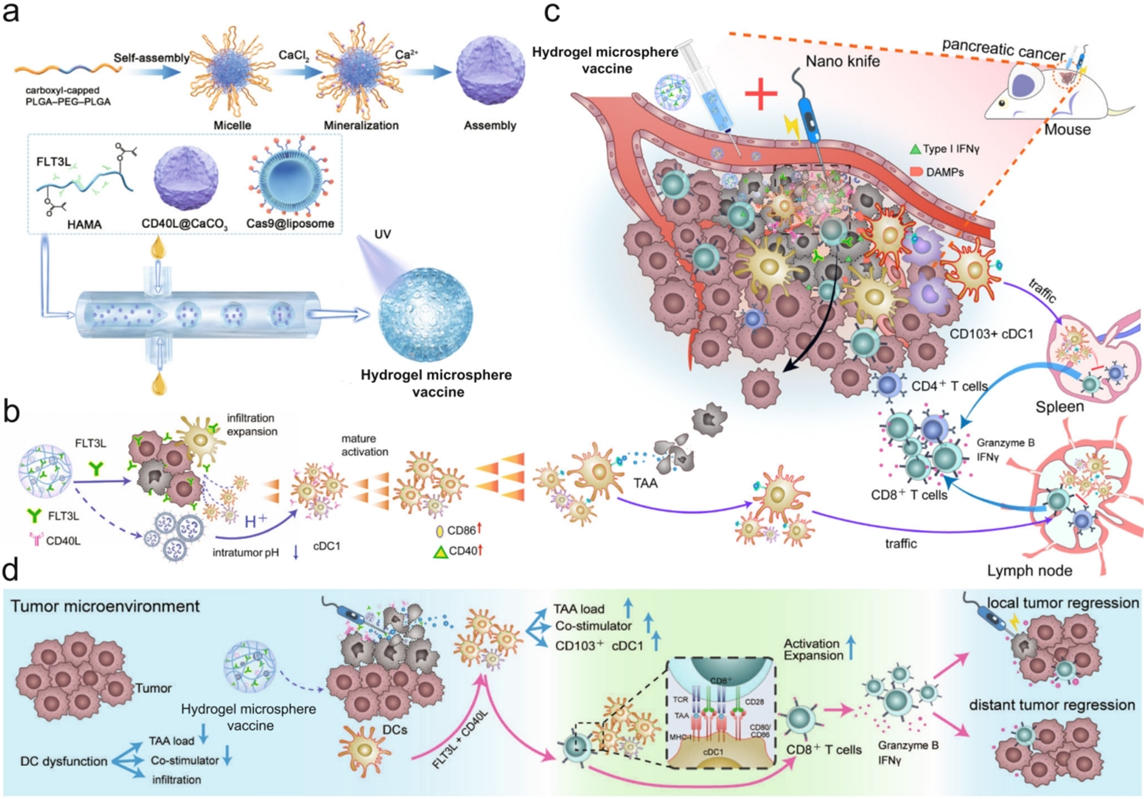
Figure 2. The cDC1-activated hydrogel microsphere vaccine was used as a general immune amplifier to amplify the cDC1/CD8+ T-cell antitumour axis after ablation therapy.
3. Immune Response Assessment
- Procedure:
- Flow cytometry was utilized to analyze immune cell populations in tumor-draining lymph nodes (TdLNs) and spleens after treatment.
- Key markers such as CD8, CD44, and IFN-γ were assessed to evaluate T cell activation and proliferation.
- Result:
- The hydrogel vaccine treatment resulted in a 20% increase in CD8+ T cell density in TdLNs and a significant increase in the proportion of activated (Ki67+ and IFN-γ+) CD8+ T cells.
- New Finding:
- The vaccine not only enhanced the local immune response but also promoted systemic anti-tumor immunity, evidenced by increased circulating CD8+ T cells and cytokine levels.
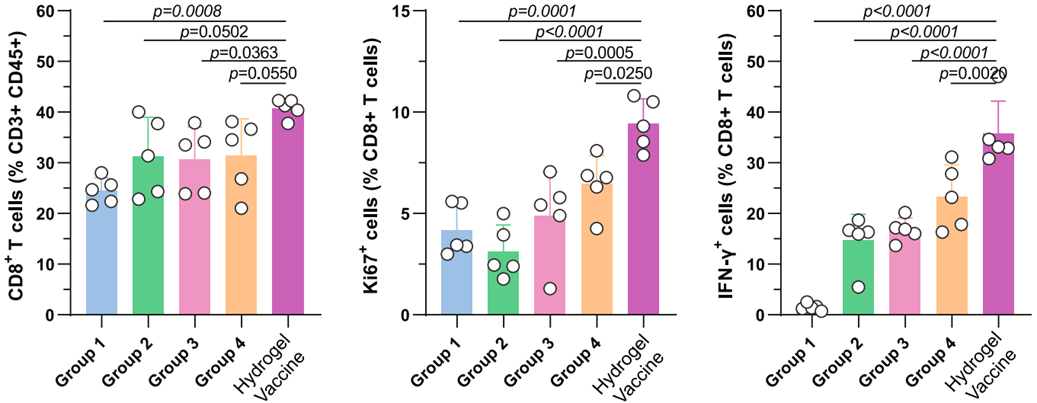
Figure 3. Identification of the proportion of CD8+ T cells (left), Ki67+ CD8+ T cells (middle) and IFN-γ+ CD8+ T cells in the TdLNs.
4. Survival Analysis
- Procedure:
- Mice were monitored for overall survival following the different treatment regimens, comparing the hydrogel vaccine combined with IRE to control groups.
- Result:
- The median overall survival for mice treated with the hydrogel vaccine and IRE was 48 days, compared to 36 days for IRE alone and 19 days for untreated controls.
- New Finding:
- This marked improvement in survival highlights the potential of the hydrogel microsphere vaccine as a potent adjunctive therapy following ablation in pancreatic cancer treatment.
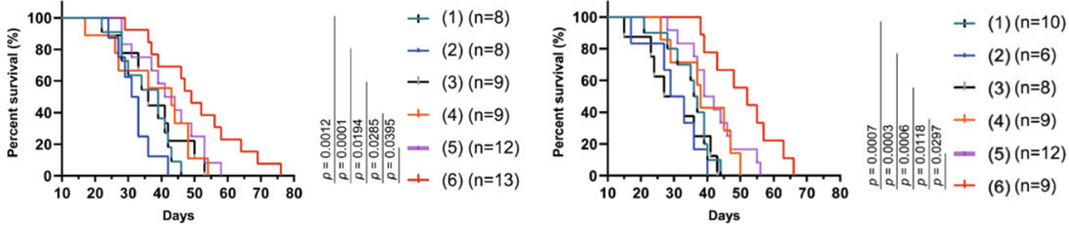
Figure 4. Survival analysis of mice bearing orthoptic tumours after different combination therapies.
These experiments collectively demonstrate the innovative approach of utilizing a hydrogel microsphere vaccine to amplify anti-tumor immunity and improve therapeutic outcomes in pancreatic cancer after ablation therapy.
Transformative Impact of Hydrogel Vaccine
The study showed that the hydrogel microsphere vaccine activates cDC1-mediated anti-tumor immunity in preclinical models of pancreatic cancer. By increasing the recruitment, maturation, and migration of cDC1 cells, the vaccine enhances the CD8+ T cell response, resulting in improved control of tumor growth and metastasis. These findings are significant as they provide a potential method to counteract the immunosuppressive barriers in PDAC, which could lead to better outcomes for patients. The use of a pH-responsive hydrogel system to deliver immune modulators directly to the tumor site represents a targeted approach that may have applications beyond pancreatic cancer, potentially extending to other forms of cancer immunotherapy. The research highlights the importance of integrating ablation therapy with immune-enhancing strategies to address the persistent challenge of tumor recurrence.
Reference:
Liu, Xiaoyu, et al. “Interventional hydrogel microsphere vaccine as an immune amplifier for activated antitumour immunity after ablation therapy.” Nature Communications 14.1 (2023): 4106.
