Editor: Nina
Researchers introduce UB-VV100, an innovative lentiviral vector platform for in vivo CAR T-cell engineering that effectively targets B-cell malignancies while eliminating the need for ex vivo manufacturing and lymphodepleting chemotherapy.
Key Preview
Research Question
The study investigates the challenges associated with the ex vivo manufacturing process of Chimeric Antigen Receptor (CAR) T-cell therapies, particularly focusing on the need for a more efficient method to generate CAR T-cells directly within the body.
Research Design and Strategy
The research employs a preclinical proof-of-concept design to evaluate VivoVec, a lentiviral vector platform designed for in vivo CAR T-cell engineering, specifically targeting B-cell malignancies. This approach aims to eliminate the logistical complexities of traditional methods while enhancing T-cell proliferation and persistence.
Method
The method involved administering the clinical candidate UB-VV100 to peripheral blood mononuclear cells (PBMCs) from healthy donors and B-cell malignancy patients. The study evaluated the transduction, expansion, and anti-tumor efficacy of CAR T-cells in humanized mouse and canine models.
Key Results
UB-VV100 demonstrated dose-dependent activation and transduction of T-cells in vitro, leading to selective expansion and effective elimination of malignant B-cell targets in vivo. The study showed that UB-VV100 could successfully generate functional CAR T-cells without the need for ex vivo manufacturing or lymphodepleting chemotherapy.
Significance of the Research
This research significantly advances the field of CAR T-cell therapy by introducing an innovative in vivo engineering platform that could streamline the treatment process, increase patient access, and reduce associated costs. It offers a potential solution to the limitations of current CAR T-cell therapies.
Introduction
Chimeric Antigen Receptor (CAR) T-cell therapies have emerged as a groundbreaking treatment option for relapsed or refractory B-cell malignancies, including B-cell acute lymphoblastic leukemia (B-ALL) and diffuse large B-cell lymphoma (DLBCL). These diseases are characterized by the excessive proliferation of malignant B-cells, leading to severe immune dysfunction and a poor prognosis. Despite the remarkable success of CAR T-cell therapies in clinical settings, their widespread adoption has been limited by the complexities associated with the traditional treatment approach.
The conventional strategy for delivering CAR T-cell therapies involves an ex vivo process where T-cells are collected from a patient, genetically modified to express CARs that target specific antigens on cancer cells, and subsequently expanded before being infused back into the patient. This method requires extensive handling, including cell culture, genetic manipulation, quality control, and patient-specific customization, which can lead to significant logistical challenges. These include prolonged preparation times, high costs, and potential safety concerns linked to lymphodepleting chemotherapy that patients must undergo prior to receiving CAR T-cell infusions.
These challenges often result in delays in treatment initiation and limited access to CAR T-cell therapies, especially for patients in underserved areas or those who cannot afford the high costs associated with these therapies. Furthermore, the reliance on ex vivo manufacturing can lead to variability in product quality and efficacy, complicating patient outcomes.
In light of these challenges, the innovative drug delivery strategy presented in this study focuses on in vivo CAR T-cell engineering using VivoVec, a lentiviral vector platform. This novel approach allows for the direct generation of CAR T-cells within the patient’s body, thereby circumventing the logistical burdens of traditional manufacturing methods. By enabling the administration of CAR T-cell therapies without the need for ex vivo processing, this strategy aims to enhance patient access, reduce treatment costs, and improve overall therapeutic efficacy, marking a significant advancement in the field of cancer immunotherapy.
Research Team and Aim
The research was conducted by a team from Umoja Biopharma, led by Dr. Kathryn R. Michels. The study was conducted in 2023 at Umoja Biopharma, a biotechnology company focused on innovative cancer therapies. The research team, which included co-authors Alyssa Sheih, Susana A. Hernandez, and others, aimed to address the challenges associated with traditional CAR T-cell therapies. Their findings were published in the paper titled “Preclinical proof of concept for VivoVec, a lentiviral-based platform for in vivo CAR T-cell engineering” in the Journal for ImmunoTherapy of Cancer.
The primary aim of the research, as articulated by Dr. Michels, was to develop and validate a platform capable of generating functional CAR T-cells directly within the patient, thereby eliminating the need for ex vivo cell manipulation. This innovative approach seeks to streamline the treatment process and enhance patient access to CAR T-cell technologies, ultimately improving outcomes for patients with B-cell malignancies.
Experimental Process
Primary Technique
The primary technique employed in this research is the use of VivoVec, a lentiviral vector platform designed for in vivo CAR T-cell engineering. This innovative approach aims to generate functional CAR T-cells directly within the patient’s body, thereby bypassing the complexities associated with traditional ex vivo manufacturing methods.
Experiment 1: In Vitro Transduction of PBMCs
Key Steps:
- Cell Preparation: Peripheral blood mononuclear cells (PBMCs) were isolated from healthy donors and patients with B-cell malignancies.
- Transduction: PBMCs were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 50 IU IL-2. UB-VV100 was added directly to the cell cultures at a multiplicity of infection (MOI) of 5 for 3 days.
- Rapamycin Treatment: On day 3 post-transduction, cells were treated with varying concentrations of rapamycin (1–40 nmol) to assess the impact on CAR T-cell expansion.
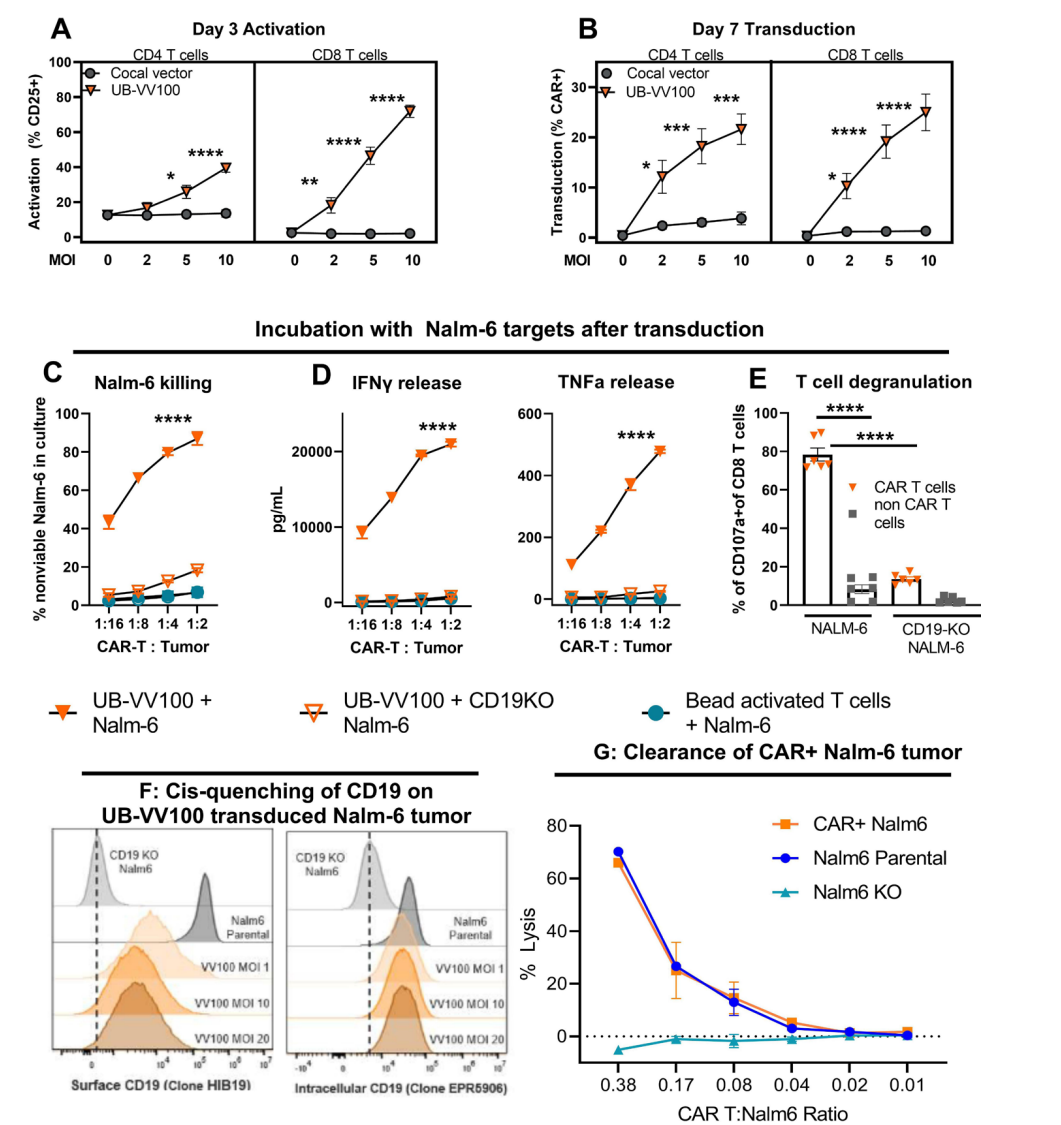
Figure 1. UB- VV100 activates and transduces unstimulated T cells. UB- VV100 is added directly to cultured PBMCs in the presence of IL- 2 and no additional stimulation. Activation, transduction, and rapamycin-mediated expansion ar e evaluated in the wells.
Data Collection and Analysis:
Data on T-cell activation and transduction efficiency were collected using flow cytometry to measure CAR expression and activation markers (e.g., CD25) on days 3 and 7. Statistical analyses were performed using two-way ANOVA to compare different treatment groups.
Novel Aspects:
This experiment highlights the use of a single-step transduction process that allows for direct generation of CAR T-cells from PBMCs without prior activation. The incorporation of rapamycin to drive selective expansion of CAR T-cells presents an advantage over traditional methods that require extensive ex vivo manipulation and lymphodepletion.
Experiment 2: RACR-mediated Expansion
Key Steps:
- Transduced Cell Culture: UB-VV100-transduced PBMCs were cultured in the presence of rapamycin at varying concentrations (0, 1, 4, 10, and 40 nM).
- Monitoring Expansion: The frequency and total number of CAR T-cells were assessed over a period of 21 days to determine the effectiveness of the RACR system in promoting T-cell proliferation.
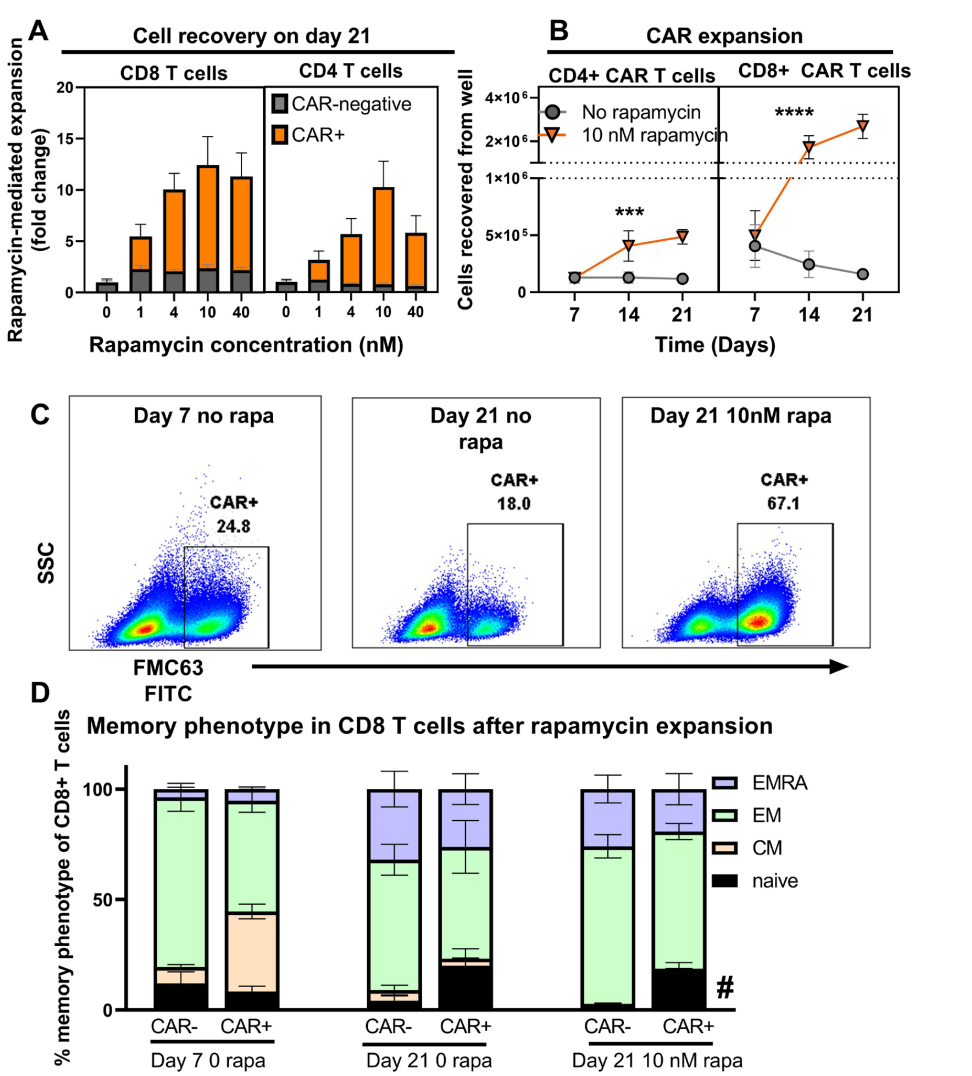
Figure 2. Rapamycin promotes selective expansion of CAR T cells.
Data Collection and Analysis:
Flow cytometry was used to quantify the number of CAR+ CD4 and CD8 T-cells, and data were analyzed using two-way ANOVA to evaluate the effects of rapamycin on cell expansion over time.
Novel Aspects:
The use of the rapamycin-activated cytokine receptor (RACR) system represents a novel approach for enhancing T-cell proliferation in vivo, allowing for the selective survival and expansion of CAR T-cells while suppressing non-transduced cells. This contrasts with traditional methods that do not efficiently support T-cell growth post-transduction.
Experiment 3: In Vivo Efficacy in Humanized Mouse Models
Key Steps:
- Model Preparation: CD34-humanized mice were engrafted with B-cell tumors and treated with UB-VV100 via intraperitoneal injection.
- Monitoring Efficacy: Blood samples were collected weekly to evaluate circulating CAR T-cells and B-cell depletion.
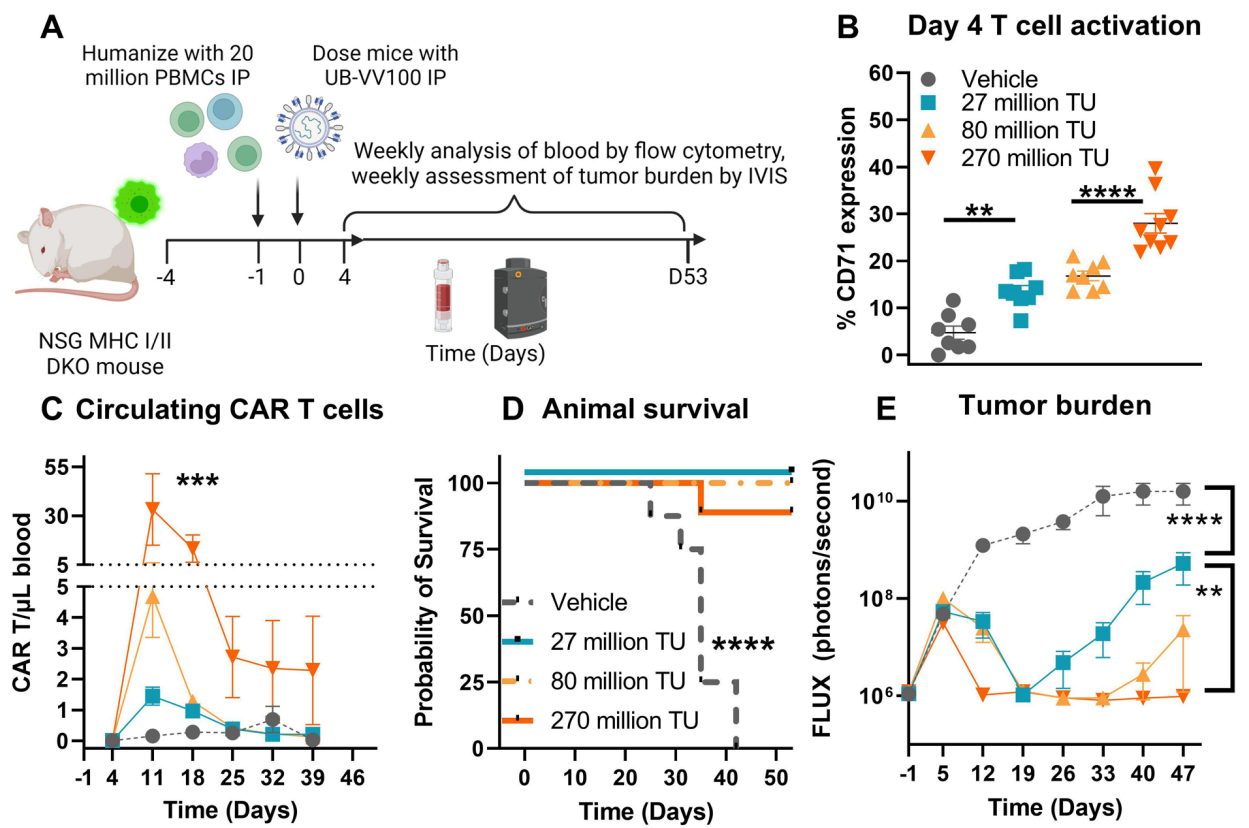
Figure 3. UB- VV100 treatment results in in vivo transduction of CAR T cells and clearance of Nalm-6 tumor.
Data Collection and Analysis:
Flow cytometry was utilized to assess the levels of B-cells and CAR T-cells in the blood. Statistical analysis was performed using two-way ANOVA to determine the significance of the findings.
Novel Aspects:
This experiment demonstrates the in vivo efficacy of UB-VV100 in generating functional CAR T-cells capable of targeting and eliminating tumors, showcasing the potential of in vivo CAR engineering. This approach avoids the need for lymphodepletion and offers a more streamlined treatment regimen compared to conventional CAR T-cell therapies.
Experiment 4: Canine Model Evaluation
Key Steps:
- Treatment Administration: Beagle dogs were administered UB-VV100 via intranodal or intraperitoneal injection.
- Biodistribution Analysis: Blood and tissue samples were collected post-injection to analyze the distribution of transduced cells.
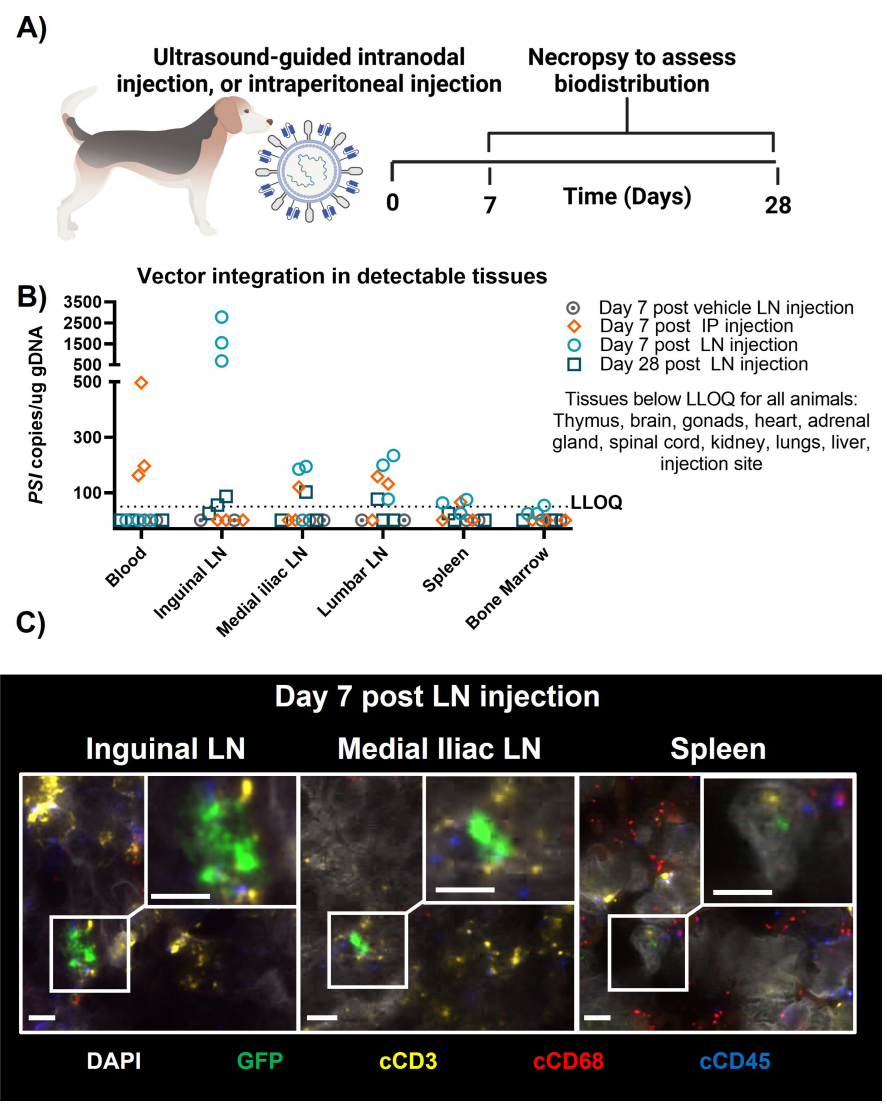
Figure 4.Biodistribution of surface- engineered lentiviral vectors in a canine.
Data Collection and Analysis:
Genomic DNA was extracted from collected samples and analyzed using qPCR to quantify lentiviral integration events. In situ hybridization (ISH) was also employed to characterize transduced cell types in tissue samples.
Novel Aspects:
The use of canine models provides a unique opportunity to evaluate the biodistribution and transduction efficiency of UB-VV100 in a large animal model, which is a significant advancement over traditional small animal models. The findings support the safety and efficacy of this delivery system in a biologically relevant context.
Overall, this research employed an innovative in vivo engineering approach that enhances the accessibility and efficacy of CAR T-cell therapies by eliminating the need for ex vivo manipulation and lymphodepletion, thereby representing a significant advancement in the field.
Conclusion
The successful development of the UB-VV100 drug delivery system was achieved through innovative engineering of a lentiviral vector, VivoVec, which allows for the in vivo generation of functional CAR T-cells directly within the patient’s body. This novel approach overcomes the limitations associated with traditional ex vivo manufacturing methods, facilitating a more efficient treatment process that enhances patient access to CAR T-cell therapies.
The highlights of the study include the demonstration that UB-VV100 effectively activates and transduces T-cells in both in vitro and in vivo settings, leading to selective expansion and efficient elimination of malignant B-cell targets. Importantly, the incorporation of a rapamycin-activated cytokine receptor (RACR) system allows for CAR T-cell proliferation in the absence of lymphodepleting chemotherapy, further streamlining the treatment process. Overall, these findings suggest that UB-VV100 has the potential to significantly improve the therapeutic landscape for patients with B-cell malignancies by reducing treatment complexities and costs while enhancing overall efficacy and safety.
Reference:
Michels, Kathryn R., et al. “Preclinical Proof of Concept for VivoVec, a Lentiviral-Based Platform for In Vivo CAR T-Cell Engineering.” Journal for ImmunoTherapy of Cancer, vol. 11, 2023, e006292.
