Editor: Tiffany
A team from Sichuan University has developed pH-responsive magnetic nanoparticles that clump inside breast cancer cells, boosting the effectiveness of heat therapy and activating the immune system.
Key Highlights
- Research Question:
Can magnetic nanoparticles (MNPs) clump inside breast cancer cells to enhance treatment? - Research Difficulties:
MNPs leaking out of tumors lower their effectiveness. - Key Findings:
MNPs clumped in acidic tumor environments, shrinking tumors by over 80% in mice. - Innovative Aspects:
MNPs aggregate only in acidic tumor conditions for better precision. - Importance of the Study:
This could lead to less toxic breast cancer treatments.
The Challenges of Breast Cancer and Magnetic Nanoparticles
Breast cancer, a leading cause of death, stems from abnormal cell growth in breast tissue and is often detected through lumps or skin changes. Current treatments like surgery, chemotherapy, and radiation face challenges such as drug resistance and severe side effects. Magnetic nanoparticles (MNPs) show potential in improving imaging and delivering targeted heat therapy (MHT) but suffer from low sensitivity and poor retention in tumors. This has driven researchers to seek innovative solutions.
Targeting Tumors with Smarter Nanoparticles
A team of scientists, led by Ao Hu and Yiyao Pu from Sichuan University, set out to tackle these limitations head-on. Their goal was to create a new kind of MNP that could overcome issues like leakage and poor distribution by aggregating—clumping together—inside tumor cells. Published in the journal Theranostics in 2023, their study focused on developing a pH-responsive MNP system. The idea was simple but clever: design MNPs that stay separate in the neutral environment of healthy tissue (pH 7.4) but clump together in the acidic conditions inside tumor cells (pH 5.5–6.8). This aggregation would trap the particles in the tumor, boosting their retention and amplifying the heat they produce during MHT. Beyond killing cancer cells directly, the team also aimed to see if this approach could wake up the immune system to fight the tumor more effectively.
The researchers had two main objectives:
- Synthesis and Design: Create MNPs of different sizes (5 nm and 20 nm) and a special pH-responsive version (called M20@DPA/HA) that changes its behavior based on pH.
- Testing Effectiveness: Study how this aggregation improves MHT, enhances tumor retention, and activates immune responses in both lab dishes and living mice with breast cancer.
From Lab to Mice: Testing the New Approach
The study involved the following experiments:
- Synthesis of MNPs (M5, M20, M20@DPA/HA)
- Characterization of MNPs
- pH-responsive aggregation tests
- Magneto-thermal conversion and MRI evaluations
- Cellular uptake and aggregation studies
- In vivo accumulation and immune activation tests
- Magnetic hyperthermia therapy in mice
Key experiments included:
- Preparation and Characterization
- Procedure: Synthesized 5 nm (M5) and 20 nm (M20) MNPs, and a pH-responsive M20@DPA/HA with an acid-reactive coating.
- Result: M5: 58.8 nm, -12.3 mV; M20: 68.1 nm, -25.6 mV; M20@DPA/HA: 295.3 nm, -41.9 mV.
- New Finding: Successfully created MNPs with distinct sizes and pH-responsive properties.
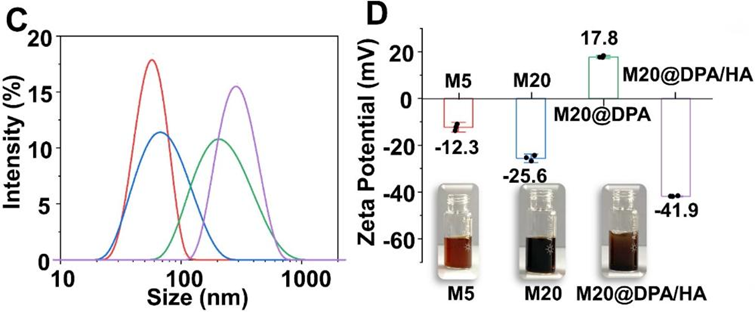
Figure 1. (C) Z-average size, zeta potential and optical photo (D) of M5, M20, M20@DPA and M20@DPA/HA, respectively.
- pH-Responsive Aggregation
- Procedure: Incubated MNPs at pH 7.4, 6.8, and 5.5.
- Result: Stable at pH 7.4; aggregated at pH 6.8 and 5.5, reaching micrometer sizes at pH 5.5.
- New Finding: Aggregation occurs in acidic conditions, ideal for tumors.
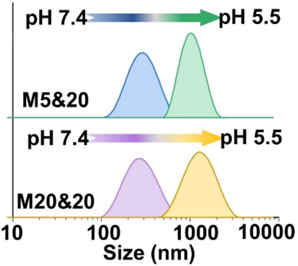
Figure 2. Size change of M5&20 and M20&20 with different pH.
- Magneto-Thermal Conversion Efficiency
- Procedure: Measured SAR and r2r2 values for aggregated MNPs (A-M20&20).
- Result: SAR: 844.4 W/g; r2r2: 465.1 mM⁻¹s⁻¹.
- New Finding: Aggregation enhances heating and imaging capabilities.
- Cellular Uptake and Intracellular Aggregation
- Procedure: Treated 4T1 cells with MNPs; measured iron content and imaged with TEM.
- Result: M20&20 had the highest iron content; TEM showed intracellular aggregation.
- New Finding: Aggregation increases MNP retention in cells.
- In Vivo Accumulation and Immune Activation
- Procedure: Injected MNPs into tumor-bearing mice; analyzed distribution and immune markers.
- Result: M20&20 penetrated 1.84 mm, covered 2.42 mm², and boosted M1 macrophages and giant cells.
- New Finding: Aggregation improves retention and activates immunity.
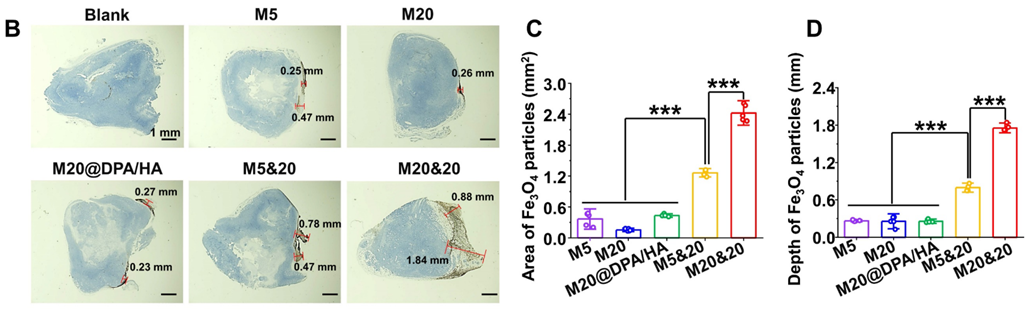
Figure 3. (B) Permeation and retention of MNPs in tumor sections by DAB-enhanced Prussian staining in different groups. Scale bars: 1 mm. (C) Retention area (mm2) and (D) permeation depth (mm) of varied MNPs in tumor sections, calculated from the images of Figure 3B.
- Magnetic Hyperthermia Therapy
- Procedure: Exposed MNP-treated mice to a magnetic field for 20 minutes.
- Result: The M20&20 group showed the most significant reduction in tumor size compared to other treatment groups in the study.
- New Finding: Aggregation greatly enhances MHT efficacy.
A Dual-Action Breakthrough for Cancer Therapy
This study marks a leap forward in cancer treatment. The Sichuan University team developed a pH-responsive MNP system that aggregates inside breast cancer cells, solving key problems like leakage and uneven spread. Their findings show this approach not only ramps up the heat delivered by MHT—killing more cancer cells—but also enlivens the immune system to join the fight. The MNPs’ improved retention, deeper tumor penetration, and enhanced imaging potential could make treatments more precise and effective.
What sets this work apart is its dual impact: directly attacking tumors while rallying the body’s defenses. By turning a weakness of MNPs—their tendency to scatter—into a strength through controlled aggregation, the researchers have opened a new path for cancer therapy. This innovation could lead to better outcomes for breast cancer patients and, with further study, other cancers too. It’s a hopeful step toward treatments that are both powerful and less punishing, offering a brighter future for those facing this relentless disease.
Reference:
Hu, Ao, et al. “Controlled intracellular aggregation of magnetic particles improves permeation and retention for magnetic hyperthermia promotion and immune activation.” Theranostics 13.4 (2023): 1454.
