Editor: Nina
Researchers develop a multifunctional nerve guidance conduit integrating a density gradient of aFGF-encapsulated collagen nanoparticles and electrospun fibers to enhance peripheral nerve regeneration and functional recovery.
Key Preview
Research Question
How can the integration of a density gradient of biomacromolecular nanoparticles with biological effectors in an electrospun fiber-based nerve guidance conduit enhance peripheral nerve repair?
Research Design and Strategy
The study utilized a multifunctional nerve guidance conduit (NGC) designed to combine topological structures, density gradients of bioactive nanoparticles, and controlled release of biological effectors to promote peripheral nerve regeneration.
Method
Researchers fabricated a graded NGC using electrospun polycaprolactone fibers and incorporated a gradient of aFGF-encapsulated collagen nanoparticles. In vitro and in vivo experiments were conducted to assess cellular proliferation, neurite extension, and functional recovery in a rat sciatic nerve injury model.
Key Results
The graded NGC significantly enhanced Schwann cell proliferation and neurite extension in vitro. In vivo, it promoted axonal elongation, remyelination, and functional recovery comparable to autografts after 6 and 12 weeks of implantation.
Significance of the Research
This research presents a novel approach to peripheral nerve repair, potentially offering a more effective alternative to traditional autografts, which often yield suboptimal recovery rates.
Introduction
Peripheral nerve injury (PNI) is a significant clinical concern characterized by damage to the nerves that can lead to debilitating motor and sensory impairments. Such injuries can arise from various causes, including trauma, surgical complications, and diseases like diabetes. The repercussions of PNI can severely diminish a patient’s quality of life, often resulting in chronic pain, loss of function, and psychological distress.
Traditionally, the standard approach to treating PNI involves nerve autografting, where a segment of healthy nerve is harvested from another part of the patient’s body and used to bridge the damaged area. This method, while effective in some cases, presents several limitations, including donor site morbidity, potential for inadequate recovery, and variable outcomes related to the size of the nerve defect. Additionally, the regeneration process can be slow and inefficient, often leading to suboptimal recovery rates.
Current strategies for drug delivery in nerve repair primarily focus on the use of growth factors, such as neurotrophic factors, to facilitate nerve regeneration. However, these factors typically have short half-lives and can be rapidly degraded in the physiological environment, limiting their therapeutic efficacy. The challenge lies in achieving a controlled and sustained release of these bioactive molecules at the site of injury to promote optimal nerve healing. Consequently, these shortcomings can lead to insufficient axonal growth, delayed functional recovery, and ultimately, poor patient outcomes.
To address these challenges, innovative drug delivery strategies are being developed, such as the incorporation of biomacromolecular nanoparticles within nerve guidance conduits (NGCs). This approach allows for the sustained release of growth factors, enhancing their local concentration and prolonging their bioactivity. By integrating topographical, haptotactic, and biochemical cues into a single scaffold, this new strategy aims to create a more conducive environment for nerve regeneration. Through these advancements, the research seeks to improve the effectiveness of PNI treatments and offer a promising alternative to traditional methods.
Research Team and Aim
The research team is led by Dr. Jian Xiao, an expert in biomedical materials and nerve regeneration, who conducted this study in collaboration with colleagues from Beijing University of Chemical Technology and Wenzhou Medical University. The research was carried out in 2022 and culminated in the publication of the paper titled “Combining a Density Gradient of Biomacromolecular Nanoparticles with Biological Effectors in an Electrospun Fiber-Based Nerve Guidance Conduit to Promote Peripheral Nerve Repair,” which was published in the journal Advanced Science.
The primary aim of the research, as articulated by Dr. Xiao, was to develop a multifunctional nerve guidance conduit (NGC) that integrates ordered topological structures, a density gradient of biomacromolecular nanoparticles, and controlled delivery of biological effectors. This innovative approach seeks to provide the necessary cues for promoting axonal elongation and enhancing functional recovery in peripheral nerve injuries.
Experimental Process
Primary Technique: Electrospinning and Coaxial Electrospraying
The main technique employed in this study is a combination of electrospinning and coaxial electrospraying. This methodology enables the creation of a multifunctional nerve guidance conduit (NGC) that integrates aligned polycaprolactone (PCL) fibers with a gradient of biomacromolecular nanoparticles encapsulating aFGF (acidic fibroblast growth factor). This combination allows for the simultaneous delivery of topographical, haptotactic, and biological cues essential for peripheral nerve repair.
Experiment 1: Fabrication of the Nerve Guidance Conduit (NGC)
- Preparation of PCL fibers: PCL was dissolved in a suitable solvent to create a 12% (wt) solution. This solution was then subjected to electrospinning to produce aligned PCL nano fibers.
- Layering: A dual-layer structure was formed, consisting of random fibers on top and aligned fibers at the bottom.
- Coaxial electrospraying: Collagen particles encapsulating aFGF were deposited onto the aligned fibers using a coaxial electrospray setup. A movable plastic mask was employed to create a density gradient of the particles across the fiber mat.
- Curing and Storage: After particle deposition, the scaffold was treated with plasma to enhance hydrophilicity and then stored at -20°C for further use.
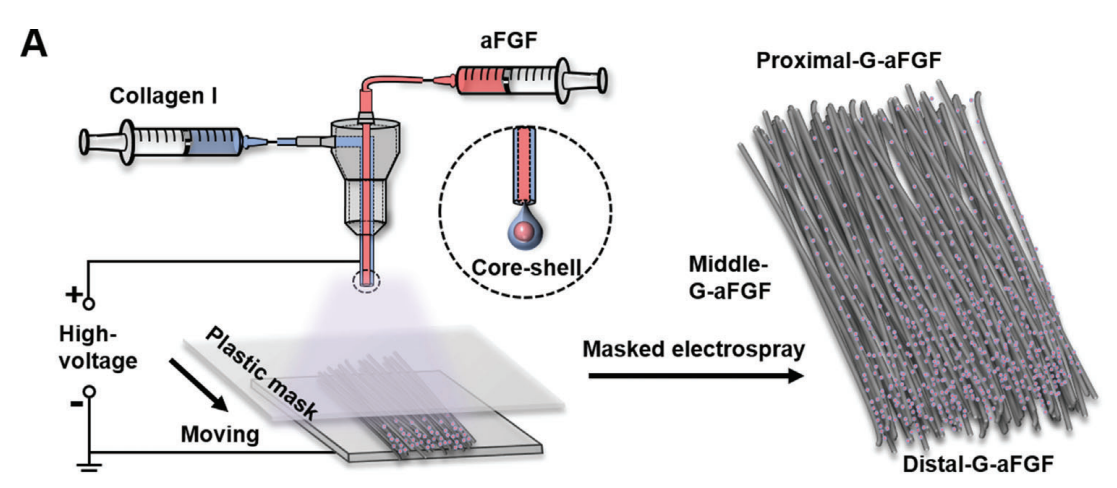
Figure 1.A) A schematic indicating the fabrication of the graded scaffold integrated with the density gradient of aFGF-encapsulated collagen particles together with uniaxially aligned electrospun nanofibers via a masked coaxial electrospraying method. The plastic mask moves to vary the collection duration of the particles along the fiber alignment.
Experiment 2: In Vitro Evaluation of Schwann Cell Proliferation
- Cell Culture: Rat Schwann cells (RSC96) were cultured on different scaffold types, including the newly developed G-aFGF scaffold and control scaffolds.
- Proliferation Assay: The proliferation of Schwann cells was assessed using a cell counting kit-8 (CCK-8) assay at various time points (1, 3, and 5 days).
- Data Collection and Analysis: Optical densities were measured using a microplate reader, and results were statistically analyzed to compare proliferation rates across different conditions.
Experiment 3: Neurite Extension from PC12 Cells and Dorsal Root Ganglia (DRGs)
- Cell Seeding: PC12 cells and isolated DRGs were seeded onto the scaffolds and induced to differentiate using nerve growth factor (NGF).
- Neurite Growth Measurement: After 9 days of culture, neurites extending from the cells were stained and visualized using fluorescence microscopy.
- Data Collection and Analysis: The lengths of neurites were quantified using image analysis software, and statistical comparisons were made to determine the significance of differences among scaffold types.
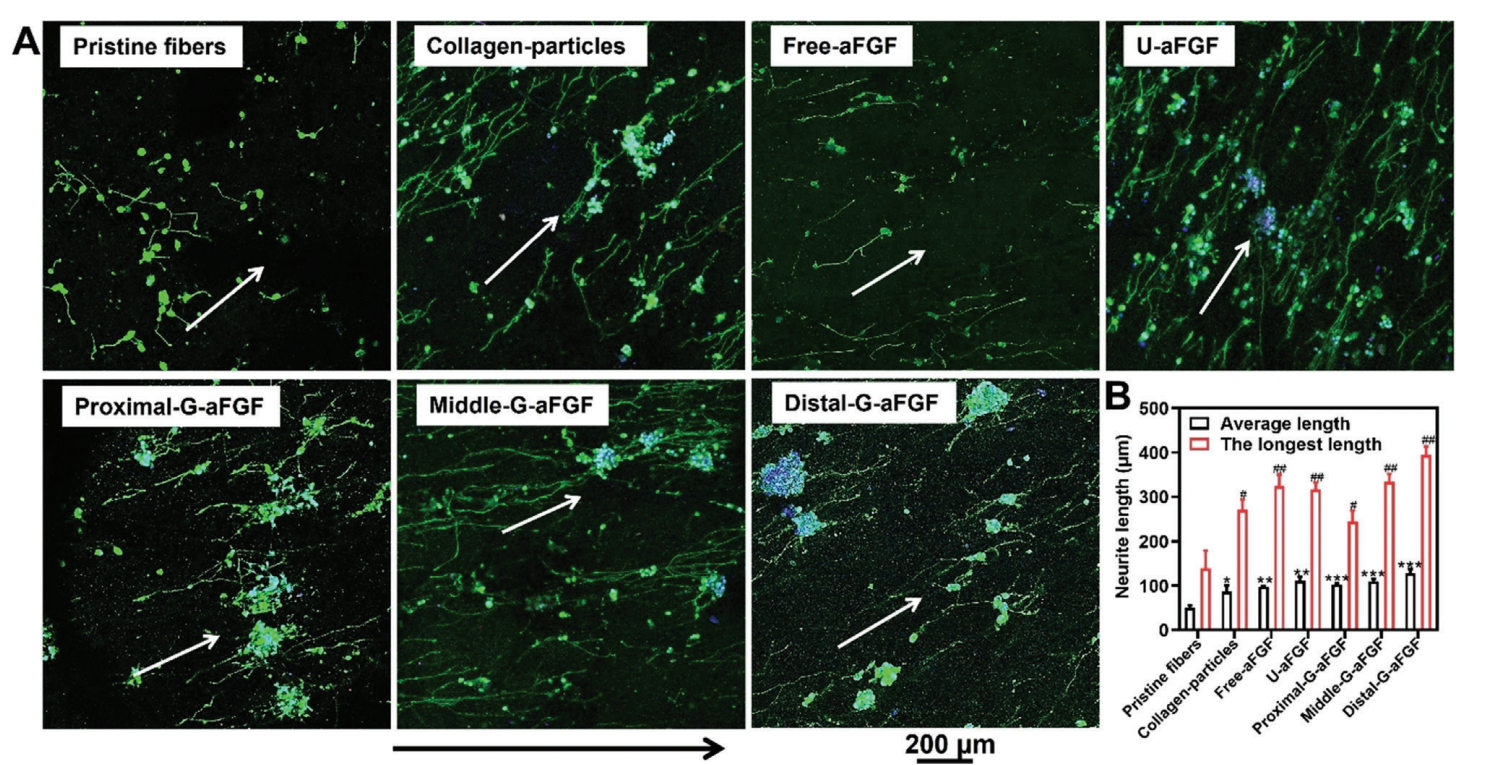
Figure 2.A) Fluorescence microscopy images of neurites extending from PC12 cells on the different scaffolds. The neurites and cell nuclei were stained with neurofilament-200 (NF-200, green) and DAPI (blue), respectively. The white arrows show the direction of fiber alignment, and the black arrow shows the gradient density of aFGF-encapsulated collagen particles from the proximal to distal position of the G-aFGF scaffold.
Experiment 4: In Vivo Evaluation of Nerve Regeneration in a Rat Model
- Animal Preparation: A total of 50 male Sprague-Dawley rats underwent surgical procedures to create a 10-mm sciatic nerve defect, which was bridged using the G-aFGF conduit or control materials.
- Post-Surgical Assessment: Functional recovery was evaluated using electrophysiological testing (measuring CMAP) and walking track analysis (calculating SFI) at 6 and 12 weeks post-surgery.
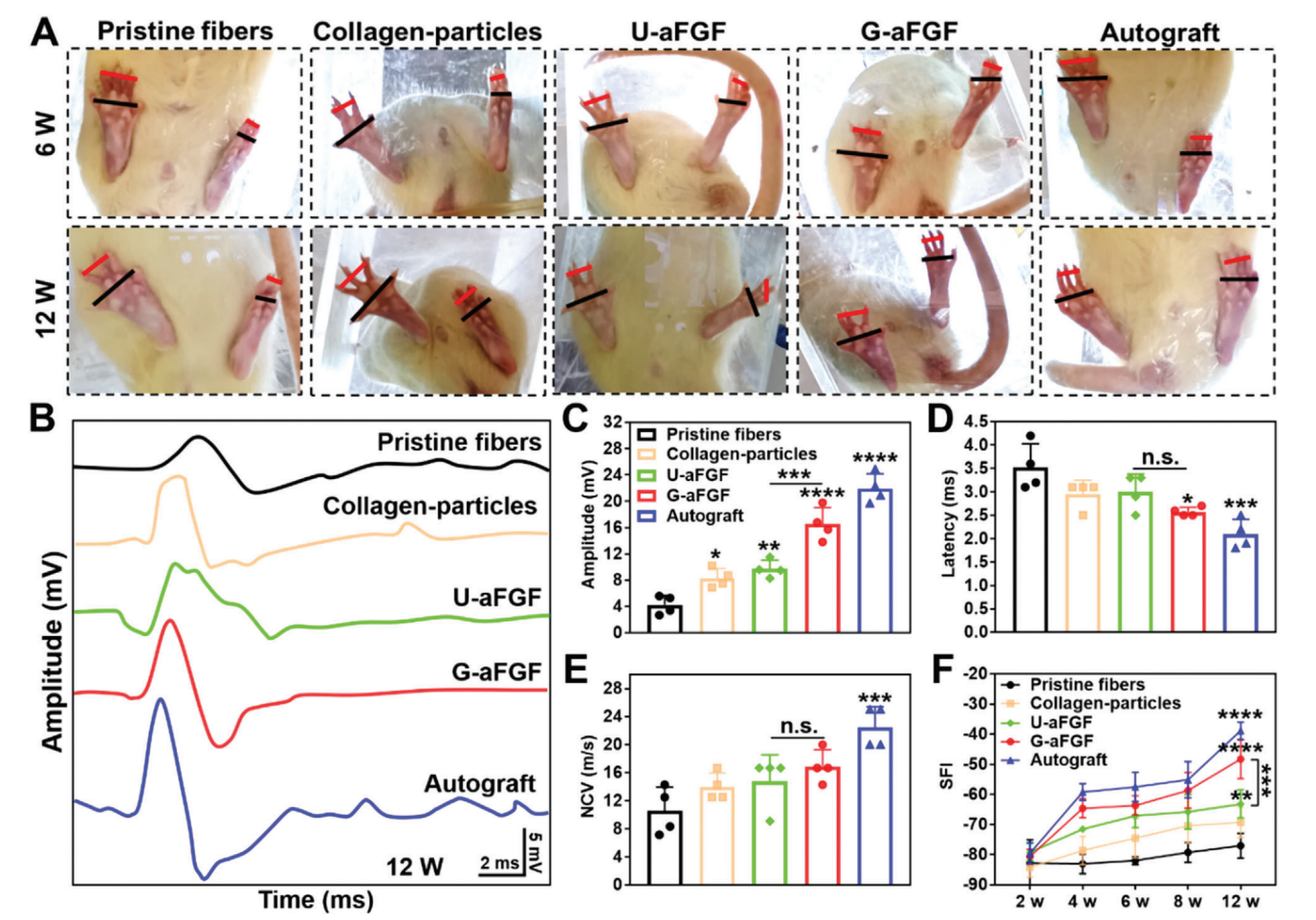
Figure 3. Functional recovery evaluation. A) Images of hind paws of the rats in five groups at 6 and 12 weeks. B) Electrophysiological assessment of CMAP. C) Quantitative analysis of CMAP amplitude. D) Quantitative analysis of CMAP latency. E) Nerve conduction velocity (NCV). F) Statistical analysis of SFI at different time points after surgery. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 (n = 4) in comparison to the group of Pristine fibers.
- Histological Analysis: At the conclusion of the study, animals were sacrificed, and nerve tissues were collected for histological examination, including hematoxylin-eosin staining and immunofluorescence for specific markers.
- Data Collection and Analysis: Functional metrics and histological data were statistically analyzed to compare the effectiveness of the G-aFGF conduit against traditional methods.
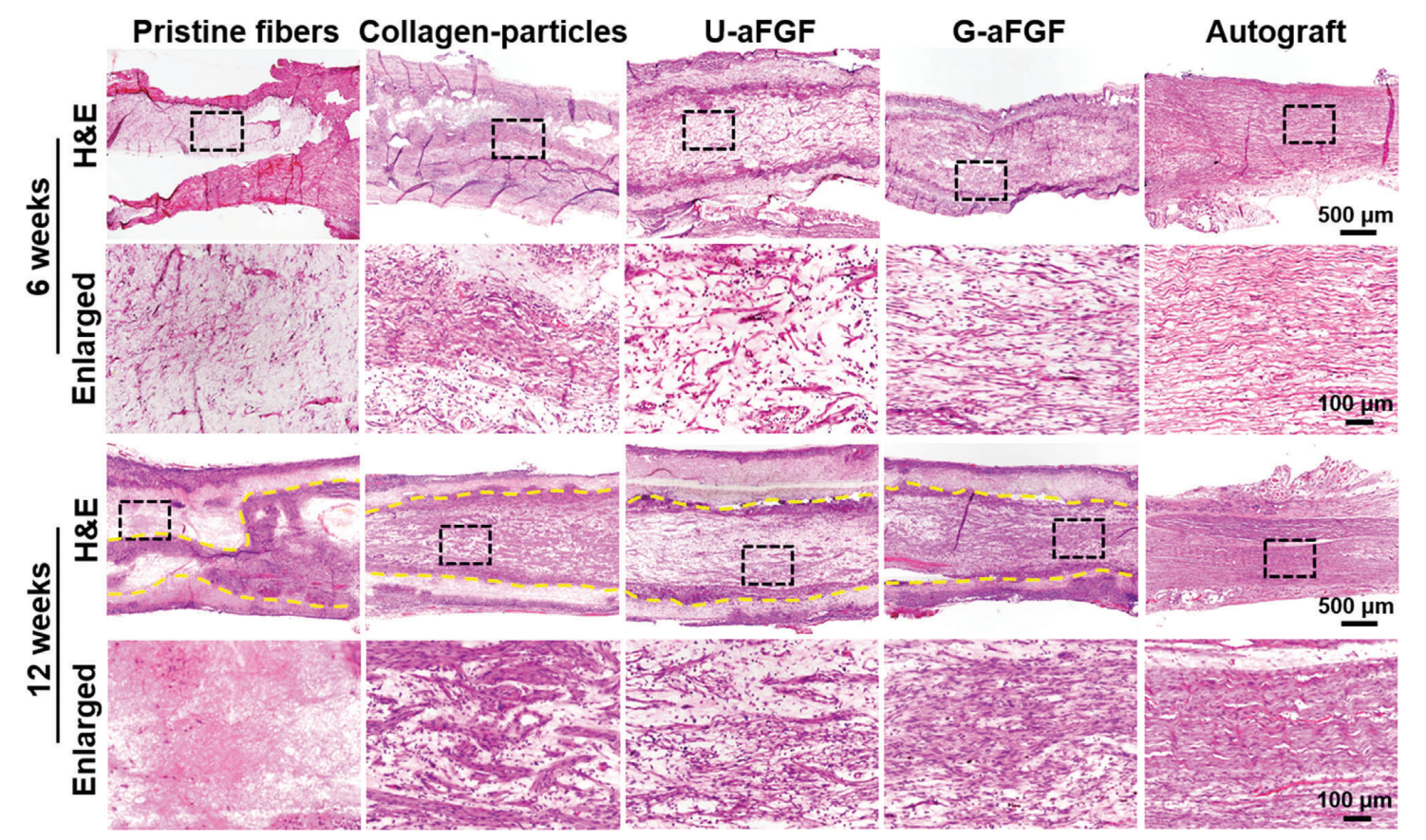
Figure 4. After surgery, H&E staining images of the longitudinal slices of regenerated nerve in five groups at 6 and 12 weeks, respectively. Dotted yellow lines indicate the edge between the scaffold and the regenerated tissue.
Novel Aspects and Advantages Over Traditional Systems
This study introduces several innovative aspects, including the use of a coaxial electrospraying technique to create a density gradient of aFGF-encapsulated collagen particles. Unlike traditional nano-delivery systems, which often deliver growth factors uniformly, this method allows for a controlled, sustained release of bioactive molecules, enhancing the local concentration of aFGF where needed most. Additionally, the combination of topographical cues from aligned fibers with biochemical signals from the nanoparticles provides a more synergistic environment for nerve repair, significantly improving outcomes compared to conventional nerve guidance conduits and autografts. This multifaceted approach not only promotes Schwann cell proliferation and neurite extension but also results in better functional recovery in vivo, addressing critical limitations of existing nerve repair strategies.
Conclusion
The successful development of this innovative drug delivery system was achieved through the integration of multiple guidance cues within a single electrospun nerve guidance conduit (NGC). By combining aligned polycaprolactone fibers with a density gradient of aFGF-encapsulated collagen nanoparticles, the researchers created a scaffold that not only provided topographical guidance for axonal growth but also facilitated controlled and sustained release of bioactive molecules. This multifaceted approach significantly enhanced the microenvironment for peripheral nerve regeneration.
The highlights of the study include the demonstration that the graded NGC markedly improved Schwann cell proliferation and neurite extension in vitro. Furthermore, in vivo assessments showed that the conduit promoted axonal elongation and remyelination, achieving functional recovery comparable to traditional autografts after 6 and 12 weeks of implantation. Overall, this research represents a promising advancement in peripheral nerve repair strategies, addressing critical limitations of existing methods and offering a more effective alternative for treating peripheral nerve injuries.
Reference
Jin, Binghui, et al. “Combining a Density Gradient of Biomacromolecular Nanoparticles with Biological Effectors in an Electrospun Fiber-Based Nerve Guidance Conduit to Promote Peripheral Nerve Repair.” Advanced Science, vol. 10, no. 2, 2023
