Editor: Nina
Scientists develop an efferocytosis-informed nanoimitator that targets synovial inflammatory macrophages to reprogram immune responses and mitigate rheumatoid arthritis symptoms.
Key Preview
- Research Question: Can an efferocytosis-informed nanoimitator (EINI) effectively reprogram synovial inflammatory macrophages (SIMs) to restore immune homeostasis and mitigate rheumatoid arthritis (RA) symptoms?
- Research Design and Strategy: The study utilized a mouse model of collagen-induced arthritis to evaluate the therapeutic effects of the EINI on inflammation and joint function.
- Method: The EINI was designed to deliver siRNA targeting the IRF5 gene specifically to SIMs in the inflamed joints, using a responsive phosphatidylserine (PtdSer) corona for macrophage uptake.
- Key Results: The EINI significantly reduced inflammation, restored joint function, and remodeled SIMs to an anti-inflammatory phenotype, demonstrating a marked reduction in proinflammatory cytokines and neutrophil infiltration.
- Significance of the Research: This innovative approach may pave the way for more effective, targeted treatments for RA, addressing the limitations of current immunosuppressive therapies.
Introduction
Rheumatoid arthritis (RA) is a prevalent and debilitating autoimmune disease characterized by chronic inflammation of the joints, leading to severe pain, swelling, and ultimately joint erosion. This condition affects millions of individuals worldwide and poses significant challenges in terms of management and quality of life. The underlying pathophysiology of RA involves the complex interplay of immune cells, particularly synovial inflammatory macrophages (SIMs), which secrete pro-inflammatory cytokines and contribute to the perpetuation of the inflammatory cycle.
Traditional treatment strategies for RA typically rely on systemic drug delivery methods, including the use of immunosuppressive medications such as corticosteroids and disease-modifying antirheumatic drugs (DMARDs). These therapies aim to reduce inflammation and slow disease progression by suppressing the immune response. However, the systemic nature of these treatments often leads to significant challenges, including increased susceptibility to infections, adverse side effects, and inadequate targeting of the inflammatory sites. Consequently, a substantial proportion of patients fail to achieve sustained remission, highlighting the need for more effective and targeted therapeutic approaches.
The current study introduces an innovative drug delivery strategy through the development of an efferocytosis-informed nanoimitator (EINI). This approach is designed to specifically target SIMs within the inflamed joints, enhancing drug localization and efficacy while minimizing off-target effects. By utilizing a phosphatidylserine (PtdSer) corona that mimics apoptotic cells, the EINI promotes selective uptake by macrophages in situ. This novel mechanism not only improves therapeutic delivery but also addresses the critical challenges associated with traditional treatments, ultimately aiming to restore immune homeostasis in RA patients.
Research Team and Aim
The research team was led by Dr. Shengchang Zhang, a prominent figure in the field of biomedical research, affiliated with Shandong University. This study was conducted over the course of several years, culminating in the publication of their findings in 2023. The paper is titled “Remodeling Articular Immune Homeostasis with an Efferocytosis-informed Nanoimitator Mitigates Rheumatoid Arthritis in Mice” and was published in Nature Communications, a prestigious journal known for its rigorous peer-review process and high-impact research.
The primary aim of the research, as articulated by Dr. Zhang, was to develop a novel therapeutic approach that could effectively reprogram synovial inflammatory macrophages (SIMs) and restore immune balance in rheumatoid arthritis (RA). The research sought to investigate whether the efferocytosis-informed nanoimitator (EINI) could significantly mitigate the symptoms of RA by targeting the underlying inflammatory mechanisms, thereby providing a more effective treatment option compared to traditional immunosuppressive therapies.
Experimental Process
Primary Technique: Development of Efferocytosis-informed Nanoimitator (EINI)
The primary technique utilized in this study was the creation of an efferocytosis-informed nanoimitator (EINI) designed to selectively target and reprogram synovial inflammatory macrophages (SIMs) in a collagen-induced arthritis (CIA) mouse model. This innovative approach leverages a phosphatidylserine (PtdSer) corona to enhance macrophage uptake and deliver siRNA that silences the IRF5 gene, addressing the inflammatory processes inherent in rheumatoid arthritis.
Key Steps of EINI Preparation
- Nanoimitator Synthesis: The EINI was synthesized using a reverse microemulsion method, where a core was formed containing dioleoylphosphatidic acid (DOPA) and metformin. This core was subsequently coated with a ROS-responsive PtdSer-NBC conjugate, designed to enhance macrophage targeting.
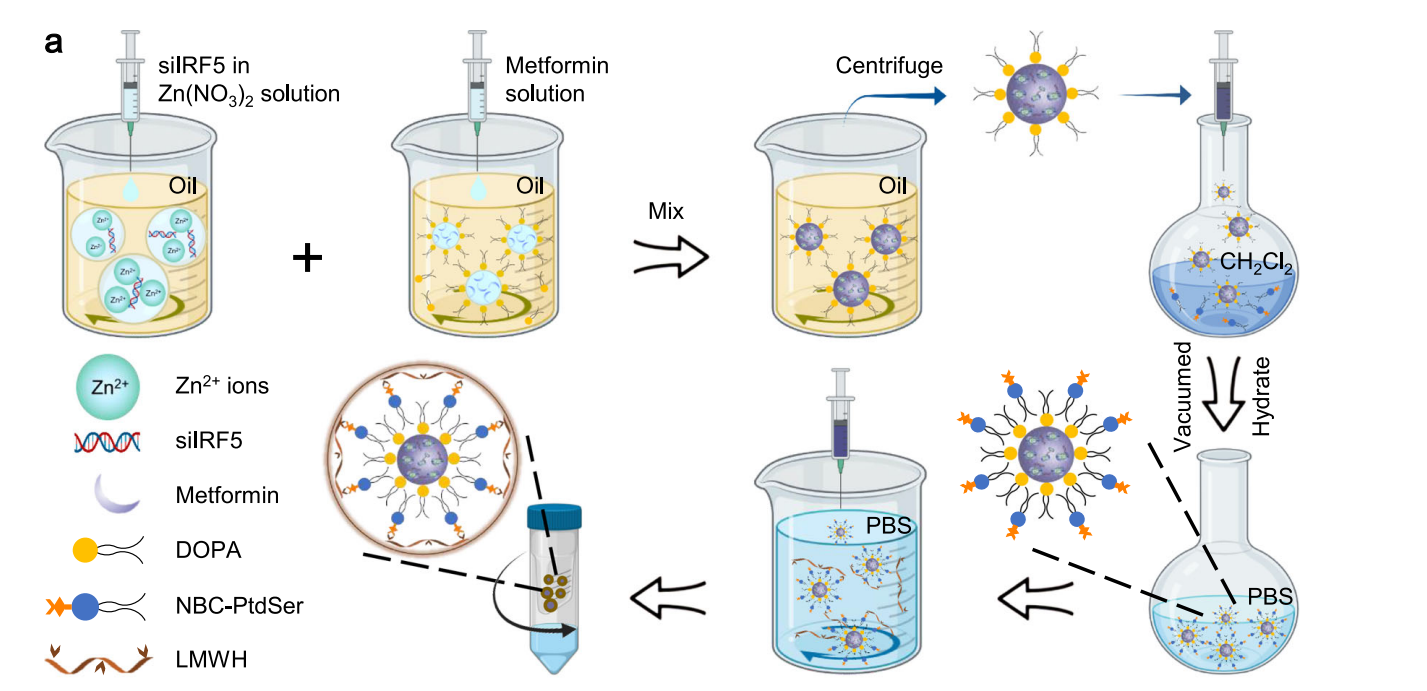
Figure 1. Schematic of the preparation of the nanoimitator by a modified reverse microemulsion method.
- Coating with Low Molecular Weight Heparin (LMWH): The next step involved cloaking the PtdSer-coated core with LMWH via a boronate ester linker. This process provided stealth characteristics for the nanoparticles in circulation and included a P-selectin-blocking motif to inhibit neutrophil trafficking to inflamed joints.
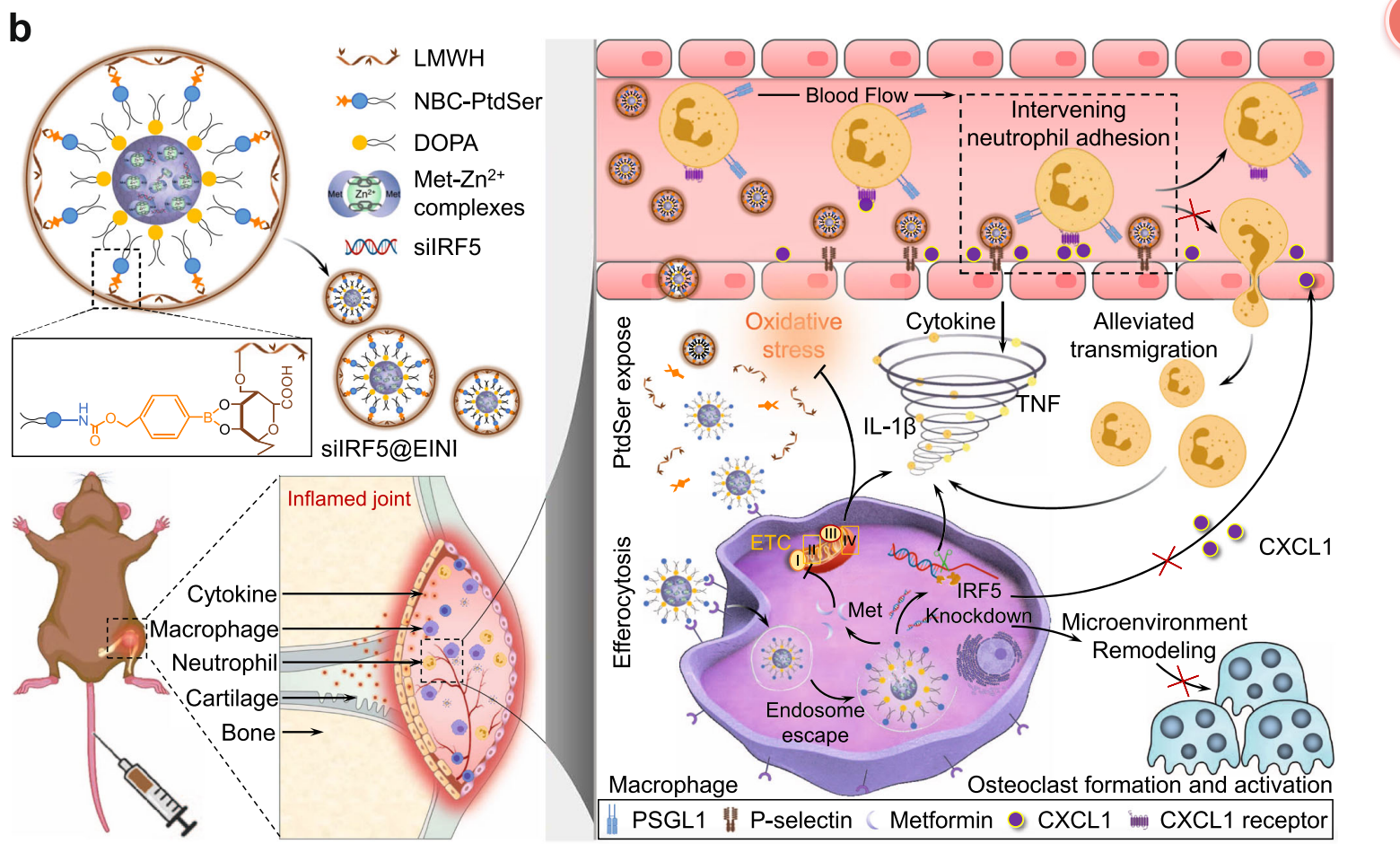
Figure 2. The siIRF5-carrying efferocytosis-informed nanoimitator (siIRF5@EINI) consisted of a drug-based core with an oxidative stress-responsive PtdSer corona and a shell composed of a P-selectin-blocking motif, low molecular weight heparin (LMWH). With the shielding of LMWH, siIRF5@EINI is endowed with stealth properties in the circulation, enhanced retention in inflamed regions, and a blocking function of P-selectin that retards the articular trafficking of neutrophils
- Characterization of EINI: Characterization of the EINI involved assessing its morphology using transmission electron microscopy (TEM) and determining size distribution and surface charge through dynamic light scattering (DLS). These evaluations confirmed the successful assembly and stability of the nanoimitator.
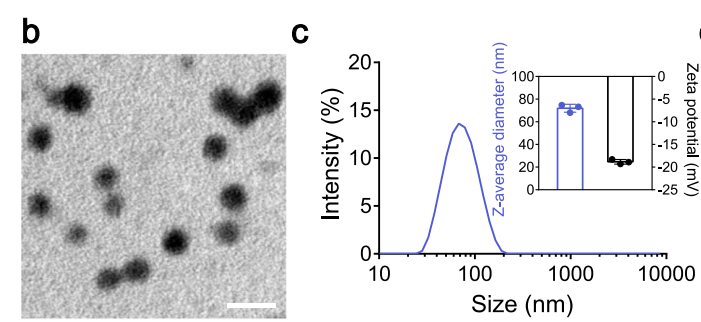
Figure 3. b. Transmission electron microscope image of siIRF5@EINI (n = 3 independent experiments). Scale bar = 100 nm. c Hydrodynamic size and zeta potential determined with DLS. Data are presented as the mean ± s.d. (n = 3 independent experiments).
Data Collection and Analysis
- In Vitro Cellular Uptake Studies: To evaluate the uptake of the EINI, fluorescence imaging was employed in activated bone marrow-derived macrophages (BMDMs) and fibroblast-like synoviocytes (FLSs). The degree of internalization was quantified using flow cytometry, allowing for precise measurement of macrophage specificity.
- In Vivo Biodistribution Assessment: For in vivo studies, CIA mice were administered the EINI, and its accumulation in inflamed joints was tracked using an IVIS imaging system. Ex vivo fluorescence analysis of major organs was performed post-administration to quantify the delivery efficiency and biodistribution of the nanoimitator.
- Gene Silencing Efficacy Measurement: The effectiveness of the EINI in silencing the IRF5 gene in macrophages was measured using quantitative real-time PCR (qRT-PCR). By quantifying IRF5 mRNA levels, the study assessed the functional impact of the targeted delivery system on inflammatory signaling pathways.
Novel Aspects
- Efferocytosis-Mimicking Design: The EINI’s design mimics the natural process of efferocytosis, enabling selective targeting and uptake by macrophages. Unlike traditional nano-delivery systems that may indiscriminately accumulate in various tissues, this specificity enhances therapeutic outcomes.
- ROS-Responsive Mechanism: The integration of a ROS-responsive PtdSer corona allows for localized activation of the EINI in the inflammatory milieu of rheumatoid arthritis. This feature enhances therapeutic efficacy while minimizing off-target effects.
- Dual Therapeutic Approach: By combining gene silencing (specifically targeting IRF5) with metabolic reprogramming through metformin, the EINI not only inhibits pro-inflammatory signals but also promotes an anti-inflammatory macrophage phenotype. This comprehensive therapeutic strategy represents a significant advancement over conventional treatments, which often rely solely on immunosuppression.
These innovative features and methodologies position the EINI as a promising therapeutic candidate for rheumatoid arthritis, potentially leading to more effective treatments with reduced side effects compared to traditional immunosuppressive therapies.
Conclusion
The successful development of the efferocytosis-informed nanoimitator (EINI) as a drug delivery system represents a significant advancement in the treatment of rheumatoid arthritis (RA). This innovative approach effectively targets synovial inflammatory macrophages (SIMs) within inflamed joints, utilizing a phosphatidylserine (PtdSer) corona that enhances macrophage uptake and facilitates localized therapeutic delivery. Through this mechanism, the EINI not only delivers siRNA to silence the IRF5 gene but also promotes an anti-inflammatory environment, addressing the root causes of inflammation in RA.
The highlights of the study include the demonstration that the EINI significantly reduces inflammation and restores joint function in a mouse model of collagen-induced arthritis. By remodeling the inflammatory macrophage phenotype and reestablishing immune homeostasis, the EINI showcases its potential as a more effective and targeted treatment option compared to traditional immunosuppressive therapies. This research paves the way for future clinical applications and opens new avenues for the treatment of other autoimmune diseases characterized by similar inflammatory processes.
Reference
Zhang, Shengchang, et al. “Remodeling articular immune homeostasis with an efferocytosis-informed nanoimitator mitigates rheumatoid arthritis in mice.” Nature Communications 14.1 (2023): 817.
