Editor: Tiffany
A new hybrid exosome system developed by Jilin University researchers enhances paclitaxel delivery, significantly suppressing colorectal tumor growth and activating the immune response in a mouse model.
Key Highlights
- Research Question:
Can hybrid exosomes improve the drug delivery capacity and therapeutic efficacy of paclitaxel in cancer therapy? - Research Difficulties:
Traditional exosome delivery systems face limitations in drug loading capacity and stability, which this study aims to overcome. - Key Findings:
The hybrid exosome-liposome system significantly enhances drug loading, stability, and therapeutic efficacy, leading to substantial tumor growth suppression in animal models. - Innovative Aspects:
This study introduces a unique fusion of mesenchymal stem cell-derived exosomes with folate-targeted liposomes to create a more effective drug delivery vehicle. - Importance of the Study:
The findings highlight a promising strategy for improving cancer treatment outcomes by enhancing drug delivery systems and modulating the tumor immune microenvironment.
Hybrid Exosomes Enhance Paclitaxel Delivery for Colorectal Cancer Therapy
Colorectal Cancer and Current Treatment Challenges
Colorectal cancer (CRC) is a significant health concern globally, characterized by its progressive nature and complex interactions with the body’s immune system. As outlined in a recent study from Jilin University, CRC involves tumor growth that can evade immune surveillance, often leading to metastasis and poor patient outcomes. The tumor microenvironment plays a critical role, with immune cells like T cells and macrophages influencing disease progression. Symptoms of CRC are not explicitly detailed in the paper but are implicitly tied to its aggressive growth and immune evasion, which complicate treatment efforts.
Current therapeutic options for CRC include paclitaxel (PTX), a plant-derived chemotherapeutic agent approved by the FDA, and its derivatives such as TAXOTERE® (1996), Abraxane® (2005), and Jevtana® (2010). These drugs target tubulin to halt cancer cell division but face significant limitations. TAXOTERE®, a paclitaxel injection, requires polyoxyethyl castor oil to improve solubility, often causing adverse reactions. Abraxane® and Jevtana®, formulated as liposomes, may still trigger allergic responses and lack precise tumor targeting, resulting in strong systemic toxicity. These challenges highlight the need for safer, more effective delivery systems to enhance paclitaxel’s therapeutic potential in CRC.
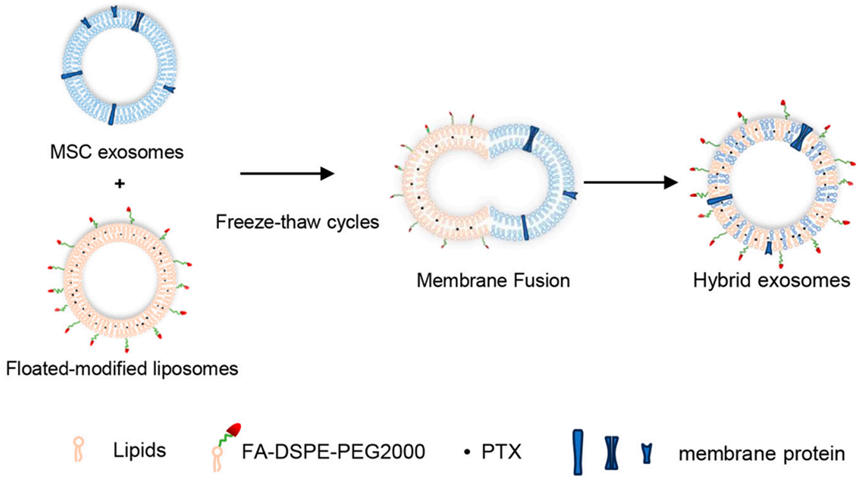
Figure 1. Hybrid exosome synthesis mechanisms.
Goals of Enhancing Paclitaxel Delivery with Hybrid Exosomes
The Jilin University study addresses key difficulties in exosome-based drug delivery, a promising yet underdeveloped approach. Exosomes, naturally occurring vesicles (30–150 nm) released by cells, offer low immunogenicity and the ability to target specific tissues but suffer from low drug loading efficiency and poor stability. These limitations reduce their clinical viability for delivering drugs like paclitaxel. The researchers aimed to overcome these hurdles by developing a hybrid exosome system that fuses mesenchymal stem cell (MSC)-derived exosomes with folate-targeted liposomes. Their main objectives were to improve drug loading capacity, enhance stability, and achieve better tumor targeting, while also evaluating the system’s therapeutic efficacy and its impact on the tumor immune microenvironment in CRC.
The research team, led by Xuan Wang and Yan Chen, included Dongdong Li, Gaotian Li, Jinda Chen, Yi Yang, Lijun Bian, Jingying Zhou, and Yongge Wu, all affiliated with the National Engineering Laboratory for AIDS Vaccine and the Key Laboratory for Molecular Enzymology and Engineering at Jilin University, China. Their findings were published on 25 March 2024 in the International Journal of Molecular Sciences.
Experimental Approach and Outcomes of the Hybrid System
The study employed a multi-step experimental process to develop and test the hybrid exosome system. It began with isolating exosomes from MSCs via ultracentrifugation, followed by preparing folate-modified liposomes using a thin-film hydration method. These components were fused into hybrid exosomes (EL) using a freeze-thaw technique, then loaded with paclitaxel (ELP). The system’s performance was assessed through in vitro cellular uptake studies and in vivo experiments using a CT26 tumor-bearing mouse model.
Three key experiments stood out:
1. Fusion Efficiency Testing
- Procedure: The team tested fusion efficiency between MSC-derived exosomes and liposomes (with positive or negative charges) at ratios of 1:1, 1:2, 1:4, and 1:6 using fluorescence resonance energy transfer (FRET) technology.
- Result: Cationic liposomes at a 1:4 ratio achieved maximum fusion efficiency (p<0.05), outperforming anionic liposomes.
- New Finding: This identified the optimal conditions for preparing hybrid exosomes, ensuring effective integration of exosomes and liposomes.
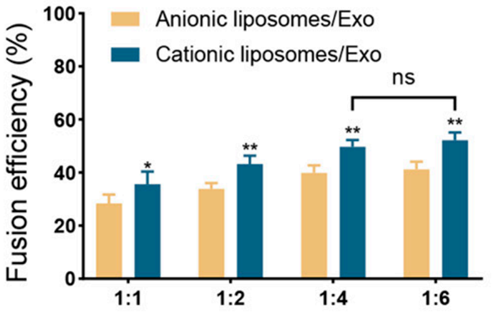
Figure 2. The fusion efficiency of Exo and liposomes with different surface charges by FRET.
2. Drug Loading and Stability Assessment
- Procedure: Paclitaxel loading was measured using high-performance liquid chromatography (HPLC), and stability was evaluated over four weeks at 4°C, with particle size monitored via dynamic light scattering (DLS).
- Result: The hybrid exosomes (EL) achieved a 2.2% loading capacity, compared to 1.0% for standalone exosomes (Exo). EL maintained stable particle sizes (~150 nm), while Exo sizes increased from 85.4 nm to 126.9 nm over time.
- New Finding: The hybrid system significantly enhanced drug loading capacity and stability, addressing a major limitation of traditional exosomes.
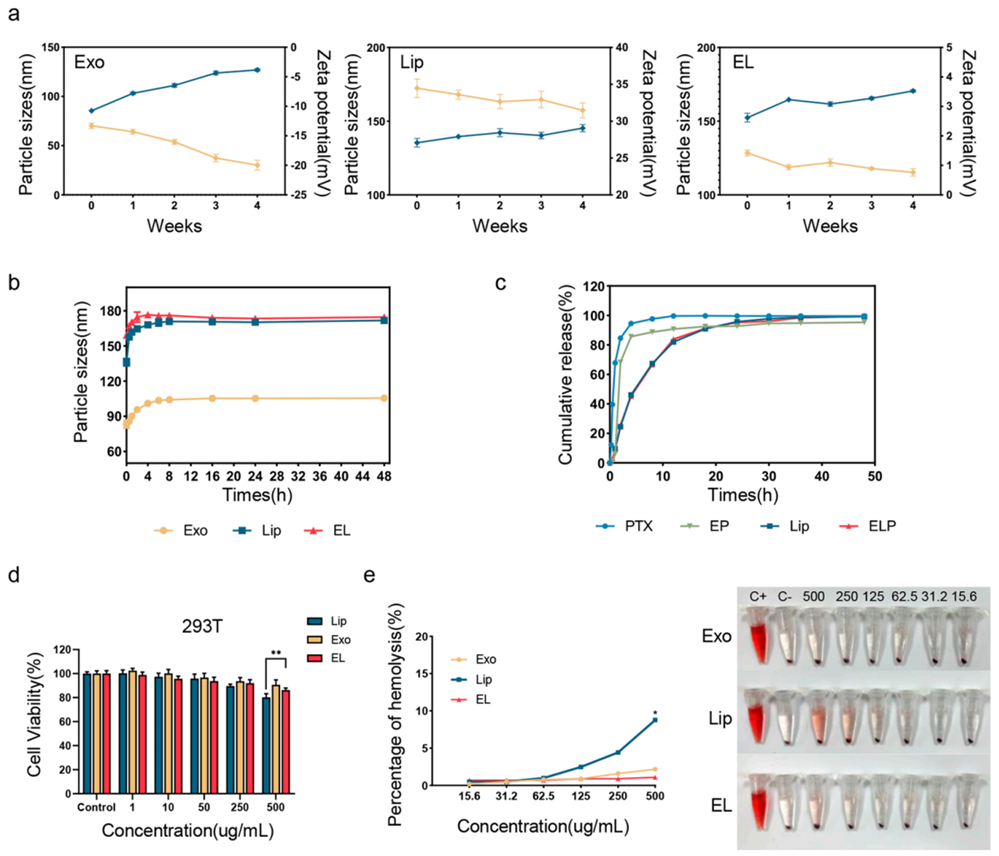
Figure 3. The stability, drug release, and safety of Exo, Lip, and EL.
3. In Vivo Antitumor Efficacy
- Procedure: CT26 tumor-bearing mice received intratumoral injections of ELP (6 mg/kg PTX) every three days for four doses. Tumor growth and survival were tracked, and immune responses analyzed via flow cytometry.
- Result: ELP significantly reduced tumor growth compared to free PTX and unloaded EL, with tumor volumes notably smaller by day 16. Survival was prolonged in the ELP group, with no significant body weight changes or organ damage across groups.
- New Finding: ELP not only suppressed tumor growth but also modulated the tumor immune microenvironment, enhancing its therapeutic impact.
Additional in vitro studies confirmed that ELP improved cellular uptake in tumor cell lines (A2780, B16, CT26) and increased apoptosis compared to free PTX, validating its targeting and efficacy.
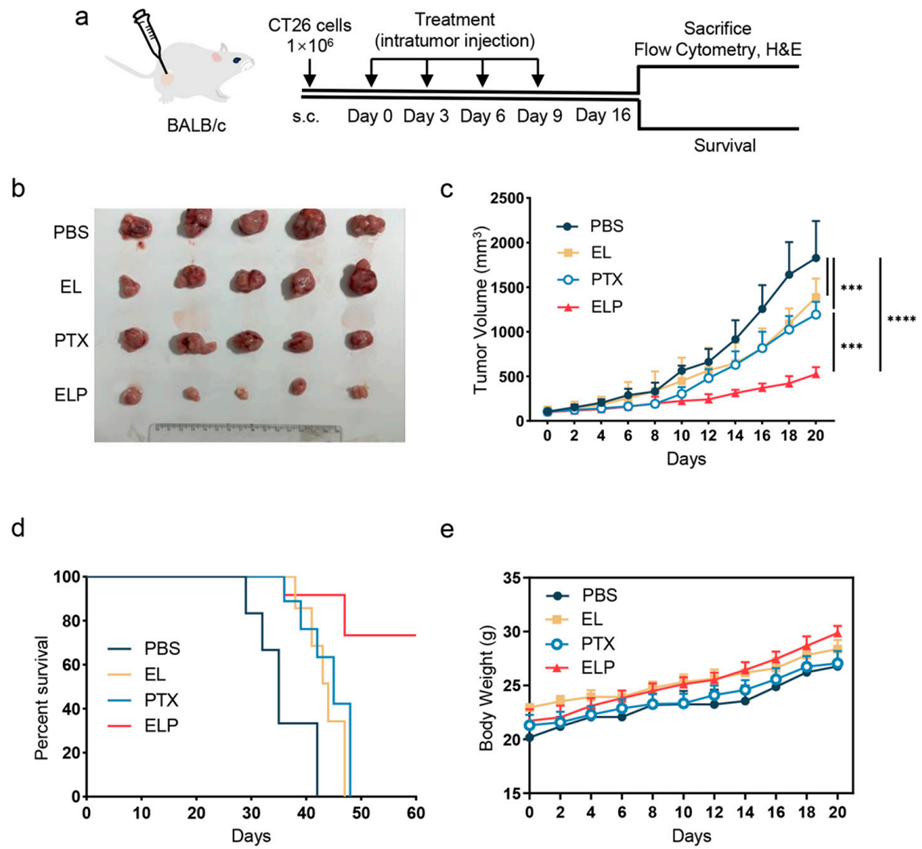
Figure 4. In vivo antitumor effects of ELP.
Key Advances in Tumor Suppression and Immune Modulation
The study demonstrates that the MSC-derived exosome-liposome hybrid system (ELP) enhances paclitaxel delivery for colorectal cancer treatment. By achieving a drug loading capacity of 2.2%, maintaining stability over weeks, and improving tumor targeting via folate modification, ELP outperformed traditional exosomes (1.0% loading capacity) and free PTX. In the CT26 mouse model, it significantly suppressed tumor growth and extended survival, underscoring its therapeutic potential. The significance lies in its dual action: delivering paclitaxel effectively while reshaping the tumor immune microenvironment.
A novel insight from this work is ELP’s ability to activate immune responses within the tumor. It increased populations of activated CD4+ and CD8+ T cells, shifted tumor-associated macrophages toward the anti-tumor M1 phenotype, and reduced immunosuppressive regulatory T cells (Tregs). These changes suggest that ELP could transform “cold” tumors—those with low immune activity—into more responsive states, offering a potential advancement in CRC therapy. While tested only in the CT26 model, these findings provide a foundation for further exploration of hybrid exosomes in cancer treatment, potentially improving outcomes by combining drug delivery with immune modulation.
Reference:
Wang, Xuan, et al. “Enhanced therapeutic potential of hybrid exosomes loaded with paclitaxel for cancer therapy.” International journal of molecular sciences 25.7 (2024): 3645.
