Editor: Tiffany
A new method called torque-focusing uses magnetic fields to precisely guide bacteria-based microrobots to tumor sites, improving drug delivery accuracy and reducing off-target effects in cancer treatment.
Key Highlights
- Research Question
Can magnetic fields guide magnetotactic bacteria to tumors for drug delivery? - Research Difficulties
Overcoming blood flow and immune barriers to microrobot targeting. - Key Findings
- Simulations: Torque-focusing reduces off-target motion, scalable to humans.
- In vitro: 40%+ improved target transport, off-target at passive levels.
- In vivo: Enhanced bacterial accumulation in mouse tumors (p=0.048).
- Innovative Aspects
- Pioneered torque-focusing with living microrobots for deep-tissue targeting.
- Scalable magnetic control for precise delivery.
- Validated in vivo in mice.
- Significance
- Improves cancer drug precision, may reduce side effects, with potential for human use.
Challenges in Cancer Drug Delivery
Cancer remains one of the most challenging diseases to treat, affecting millions of people globally. A key difficulty in cancer therapy is delivering drugs directly to the tumor without damaging healthy tissues. Tumors are often located deep within the body, surrounded by complex physiological barriers that make precise targeting hard to achieve. For instance, blood vessels near tumors can be irregular and leaky, allowing some drug leakage, but high pressure inside the tumor can push drugs back out. Additionally, the body’s immune system may detect and eliminate foreign substances, including drug carriers, before they reach their target.
Current cancer treatments, such as chemotherapy, can be effective but often affect both cancerous and healthy cells, leading to side effects like fatigue, nausea, and hair loss. To improve drug delivery, scientists have developed nanoparticle-based systems—tiny particles designed to carry drugs and accumulate in tumors due to their leaky blood vessels. While these systems offer some advantages over traditional methods, they are not without flaws. Studies show that only a small fraction of the nanoparticles reach the tumor, with the rest circulating elsewhere in the body, potentially causing unintended side effects. This inefficiency has driven researchers to explore new ways to enhance the precision of drug delivery.
Developing Precise Tumor Targeting with Torque-Focusing
A team of researchers, including Nima Mirkhani, Michael G. Christiansen, Tinotenda Gwisai, Stefano Menghini, and Simone Schuerle, sought to address these challenges by developing a more accurate method to guide therapeutic agents to tumors. Their study, published on March 9, 2024, in Nature Communications, focuses on using magnetotactic bacteria (MTB) as microrobots to deliver drugs. These bacteria naturally produce magnetic particles within their cells, making them responsive to magnetic fields, which can be used to steer them toward a specific location.
The primary challenge the team aimed to overcome was controlling these microrobots precisely within the body’s complex environment. Without precise control, the bacteria could end up in healthy tissues, reducing the treatment’s effectiveness and increasing side effects. To solve this, the researchers developed a technique called “torque-focusing,” which combines rotating magnetic fields (RMFs) with magnetostatic selection fields. This method allows them to direct the MTB to the tumor site while limiting their activity elsewhere. Their objective was to demonstrate that this approach could improve targeting accuracy and enhance the delivery of therapeutic agents to tumors.
Testing Torque-Focusing: From Simulations to Mouse Trials
The researchers tested their torque-focusing method through a series of experiments, ranging from computer simulations to tests in living mice. Below is an overview of their approach and findings, with detailed descriptions of the key experiments.
The process began with theoretical modeling, followed by laboratory tests in controlled environments, and concluded with validation in a mouse model. They used numerical simulations, in vitro microfluidic devices, and an in vivo apparatus designed for mice to assess the method’s feasibility and effectiveness.
Key Experiments
1. Numerical Modeling of Torque Suppression
The first step involved computer simulations to study how RMFs and selection fields affect the movement of MTB. The goal was to determine if they could suppress the rotational force (torque) on the bacteria outside a designated target area, preventing unnecessary motion in healthy tissues. The team modeled the magnetic fields’ effects on the bacteria and analyzed the resulting torque density. Their findings showed that torque could be spatially controlled, meaning they could focus the bacteria’s activity in a specific region while minimizing it elsewhere. Importantly, the simulations suggested that this approach could be scaled up for potential human applications, as the control remained effective across larger distances. This provided a foundation for the experimental tests that followed.
2. In Vitro Microfluidic Testing
Next, the researchers conducted laboratory experiments using a microfluidic device, a small system of channels designed to mimic conditions inside the body. They filled the channels with collagen gels to simulate tissue and introduced non-magnetic nanoparticles (NPs) as a stand-in for therapeutic agents. By applying RMFs and selection fields, they tested whether the torque-focusing method could enhance the transport of these particles to a target region within the device. The results showed that over 40% of the enhanced NP transport was maintained in the target area, while transport outside this region dropped to levels similar to passive diffusion (movement without external influence). This indicated that the method could concentrate particles where needed, offering a significant improvement over less targeted approach. The experiment highlighted the potential of torque-focusing to localize drug delivery effectively.
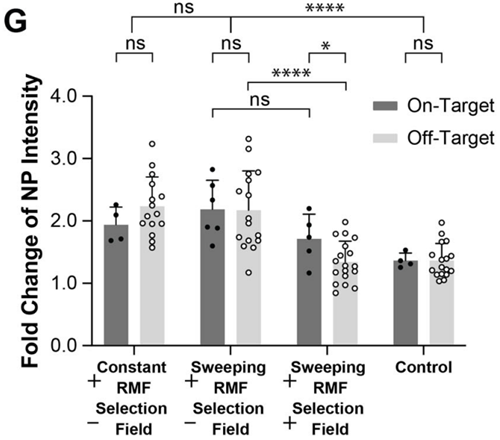
Figure 1. Fold change of NP fluorescent intensity in the collagen compartment for NP-MTB suspensions under torque-based actuation with and without a selection field and sweeping vs constant RMFs compared to unactuated, diffusion-based controls.
3. In Vivo Mouse Tumor Model
The final and most critical test involved living mice with tumors. The team injected MTB intravenously into the mice and used a custom-built apparatus to apply the torque-focusing technique. They divided the mice into three groups for comparison: one received no actuation (no magnetic fields), another received global RMF (magnetic fields applied everywhere), and the third received focused actuation (magnetic fields applied only to the target area). After treatment, they measured bacterial accumulation in the tumors. The results showed a clear difference: the focused actuation group had significantly higher bacterial presence in the tumors compared to the other groups, with a statistical significance of p=0.048. This finding confirmed that the torque-focusing method could direct the microrobots to the tumor site more effectively than unfocused or no actuation, validating the approach in a living system.
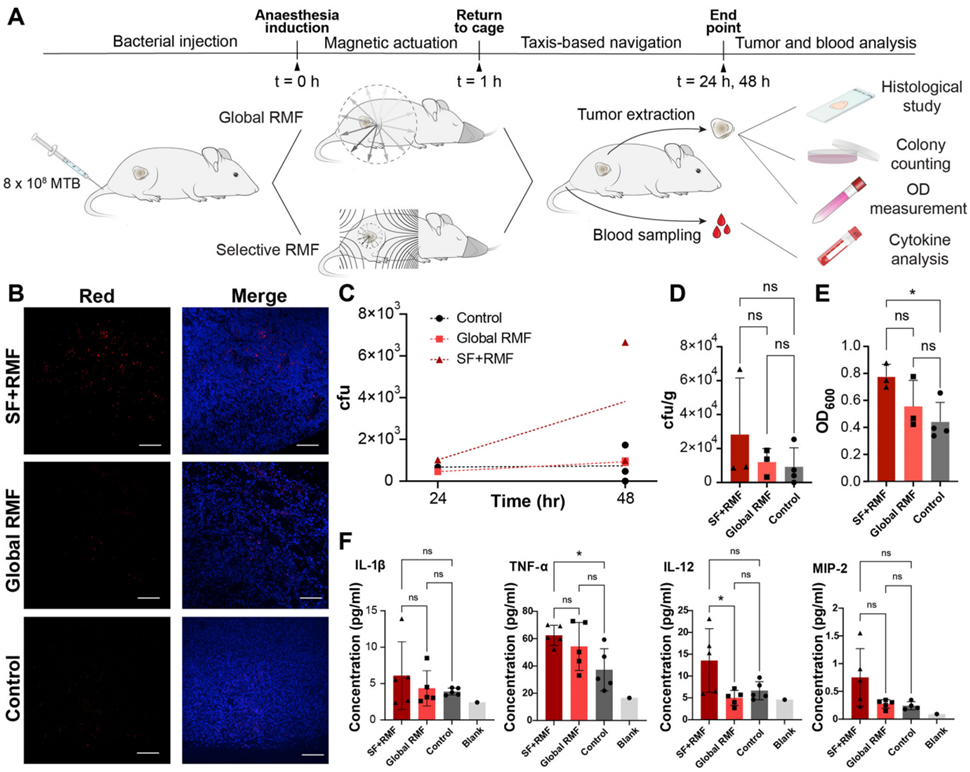
Figure 2. In vivo pilot study demonstrates the promise of torque-focusing.
Achieving Enhanced Tumor Targeting with Microrobots
The study by Mirkhani and colleagues introduces a new method called torque-focusing, which uses rotating magnetic fields and magnetostatic selection fields to steer magnetotactic bacteria to tumor sites with improved precision. Through a combination of simulations, lab tests, and mouse experiments, the team demonstrated that this technique enhances the accumulation of these microrobots in tumors while reducing their presence in healthy tissues.
This development is significant for cancer therapy because it addresses a persistent challenge: delivering drugs accurately to tumors without affecting the rest of the body. By using living microrobots—bacteria that can navigate deep tissues—and controlling them with scalable magnetic fields, the method offers a promising step toward more effective and less invasive treatments. The increased bacterial accumulation in tumors, as seen in the mouse model (p=0.048), and the ability to maintain over 40% of enhanced nanoparticle transport in vitro underscore its potential. Looking ahead, the researchers suggest that this approach could be adapted for human use, opening new possibilities for targeted cancer therapies that minimize side effects and maximize impact.
Reference:
Mirkhani, Nima, et al. “Spatially selective delivery of living magnetic microrobots through torque-focusing.” Nature Communications 15.1 (2024): 2160.
