Editor: Tiffany
Researchers have developed a new nanotechnology-based treatment that could significantly improve outcomes for women suffering from perimenopausal depression.
Key Highlights
- Research Question:
What is the potential of exosome-sheathed ROS-responsive nanogels loaded with PACAP and estrogen in treating perimenopausal depression? - Research Difficulties:
Challenges include crossing the blood-brain barrier, managing side effects of estrogen therapy, and targeting the treatment effectively to the brain. - Key Findings:
The nanogels demonstrated efficient cellular uptake and penetration of the blood-brain barrier, leading to improved behavioral outcomes in a mouse model of perimenopausal depression. - Innovative Aspects:
The use of exosome-sheathed nanogels for targeted drug delivery to the brain, responsive to reactive oxygen species, is a novel approach. - Importance of the Study:
This research could lead to more effective and safer treatments for perimenopausal depression, addressing a significant unmet medical need.
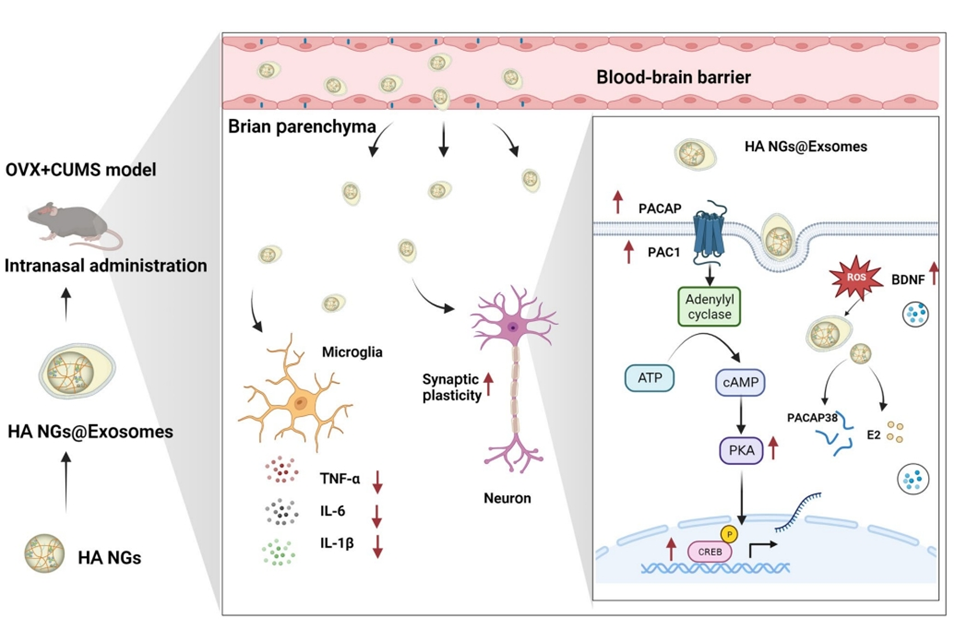
Figure 1. Graphical abstract
Perimenopausal Depression: A Growing Challenge with Limited Solutions
Perimenopause is the transitional phase before menopause, typically affecting women in their 40s and 50s. During this time, shifting hormone levels can trigger various symptoms, such as mood swings, anxiety, and depression. Perimenopausal depression is a specific form of depression tied to this period, often more intense than depression experienced at other life stages. Women may feel persistent sadness, irritability, or a lack of interest in daily activities.
Current treatments include antidepressants and hormone replacement therapy (HRT) with estrogen. However, these approaches have drawbacks. Antidepressants can take weeks to show effects and don’t work for everyone. Estrogen therapy, while sometimes effective, carries risks like increased chances of breast cancer or heart disease, especially with prolonged use. The higher prevalence of perimenopausal depression compared to other life stages highlights the urgent need for better options.
Aiming for Precision: Targeting the Brain with Nanotechnology
Led by Yue Hu, Min Zhao, and Hui Wang from Nanjing University of Chinese Medicine, this study was published in the Journal of Nanobiotechnology on August 8, 2023.
The research team aimed to develop an advanced, targeted treatment for perimenopausal depression, addressing the shortcomings of conventional therapies like antidepressants and HRT. Their primary goal was to engineer a nanotechnology-based drug delivery system capable of crossing the blood-brain barrier—a tightly regulated interface that prevents most drugs from reaching the brain—while minimizing systemic side effects. Specifically, they sought to create exosome-sheathed, ROS-responsive nanogels loaded with pituitary adenylate cyclase-activating polypeptide (PACAP) and estrogen. These nanogels were designed to release their therapeutic payload in response to the brain’s elevated reactive oxygen species (ROS) levels during depression, ensuring precise, environment-triggered drug delivery. The team also aimed to evaluate this system’s efficacy in reducing depressive symptoms and improving brain function in a mouse model of perimenopause.
From Lab to Mice: Testing a New Nanogel Therapy
Experimental Process Outline
- Modified hyaluronic acid (HA) to create HA-AT.
- Created nanogels (HA NGs) from HA-AT and loaded them with PACAP and estrogen.
- Isolated exosomes from mouse cells (Raw264.7).
- Coated the nanogels with exosomes to make HA NGs@exosomes.
- Tested the properties of HA NGs@exosomes, like size and stability.
- Conducted lab tests to see if cells could take up the nanogels and if they released the drugs properly.
- Tested in mice to see where the nanogels went in the body and if they helped with depression-like symptoms.
- Analyzed how the treatment affected inflammation, oxidative stress, and brain cell connections.
Key Experiments
1. Cellular Uptake Assay
Procedure:
Brain cells from mice were grown in lab dishes and treated with a substance (LPS) to mimic inflammation, similar to what happens in the brain during depression. The nanogels (HA NGs@exosomes) were labeled with two glowing markers: one for the exosome coating and one for the nanogel core. These labeled nanogels were added to the cells for 4 hours. Afterward, the cells were washed to remove any nanogels that hadn’t been absorbed, and then examined under a special microscope to check if the nanogels were inside the cells.
Result:
The microscope images showed that both markers were present inside the brain cells, meaning the entire nanogel structure, including the exosome coating, was successfully taken up by the cells.
New Finding:
This experiment demonstrated that the exosome coating allows the nanogels to be efficiently absorbed by brain cells, which is crucial for delivering the treatment directly to the brain.
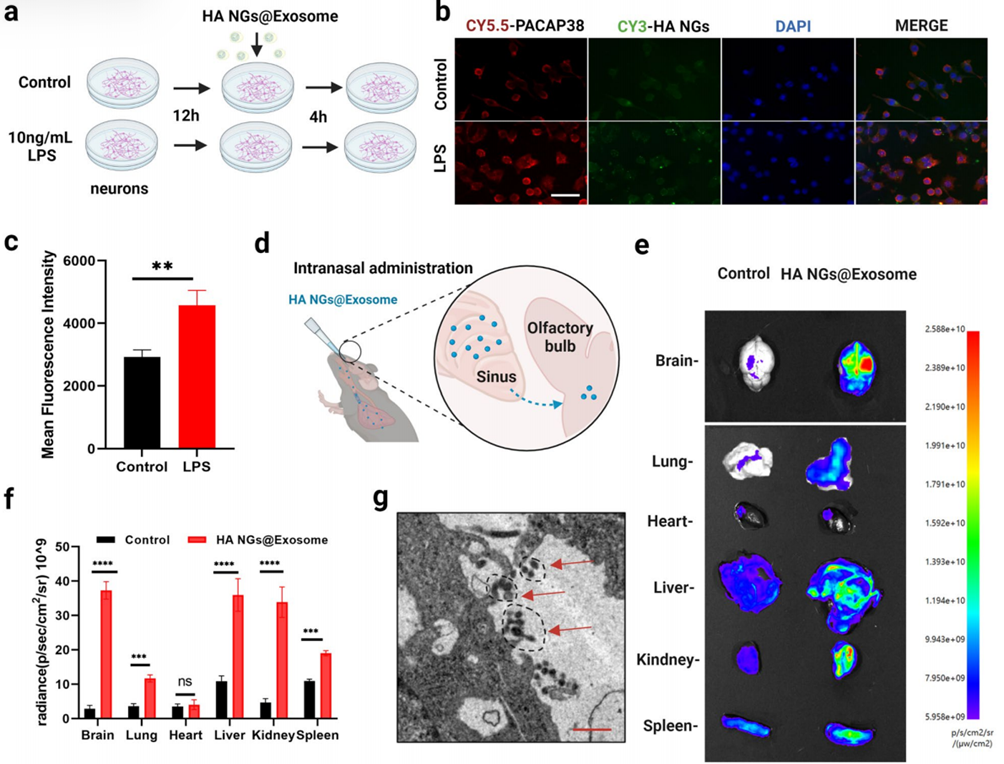
Figure 2. Cellular uptake and accumulation of HA NGs@exosomes in vitro and in vivo.
2. Behavioral Tests in Mice
Procedure:
Female mice had their ovaries removed and were exposed to ongoing stress to create a model of perimenopausal depression. The mice were divided into three groups: one received the drug-loaded nanogels (HA NGs@exosomes with PACAP and estrogen), another received empty nanogels (without drugs), and a control group received no treatment. The nanogels were administered through the nose, a method that helps drugs reach the brain more directly. After 24 hours, the mice were tested in two behavioral experiments:
- Forced Swimming Test: Mice were placed in a container of water for 6 minutes, and the time they spent floating without moving (immobile) was measured.
- Tail Suspension Test: Mice were hung by their tails for 6 minutes, and the time they spent not moving was measured.
In both tests, more immobile time suggests more depressive-like behavior.
Result:
The mice treated with the drug-loaded nanogels spent significantly less time immobile in both tests compared to the other groups, indicating they had fewer depressive-like symptoms.
New Finding:
This experiment showed that the nanogel treatment could rapidly improve behavior in a mouse model of perimenopausal depression, suggesting it might be a fast-acting therapy for this condition.
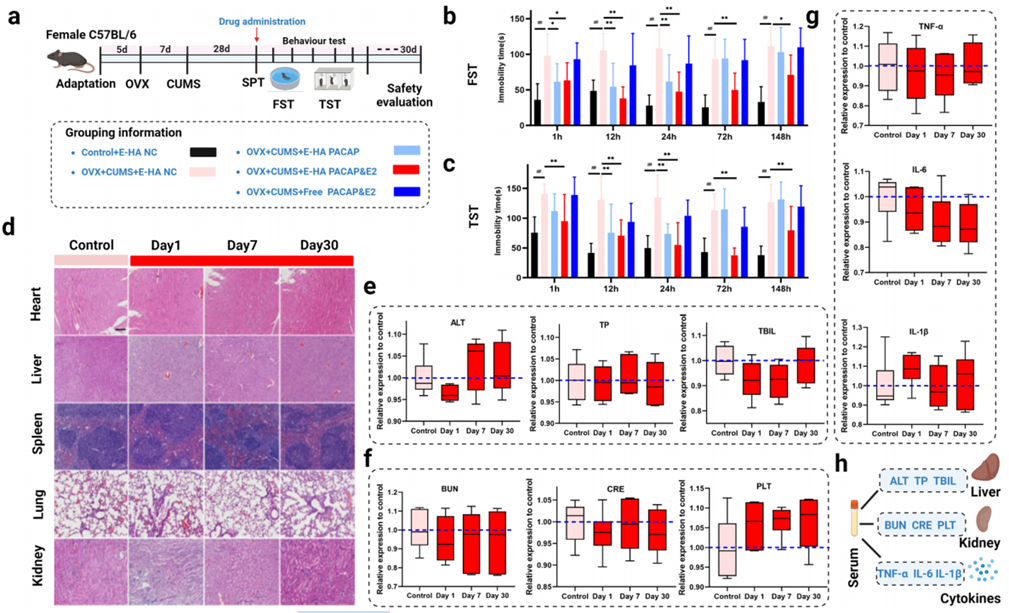
Figure 3. The effect of HA NGs@exosomes on depressive-like behaviors and its safety evaluations.
A Step Forward: Safer, Faster Relief for Perimenopausal Depression
This study presents a breakthrough in treating perimenopausal depression, demonstrating that exosome-sheathed, ROS-responsive nanogels loaded with PACAP and estrogen effectively alleviate depressive symptoms in a mouse model. The treatment targets the brain directly, crossing the blood-brain barrier to deliver drugs precisely where needed, thus reducing systemic exposure and associated risks. Key findings from the paper’s conclusion reveal that this approach not only improves mood-related behaviors but also mitigates brain inflammation and oxidative stress—hallmarks of depression linked to hormonal decline. Additionally, it enhances synaptic plasticity, the brain’s capacity to form and strengthen neural connections, which is often compromised in depressive states. Compared to traditional therapies, this nanotechnology-based method offers faster, more targeted relief with fewer side effects.
Reference:
Hu, Yue, et al. “Exosome-sheathed ROS-responsive nanogel to improve targeted therapy in perimenopausal depression.” Journal of Nanobiotechnology 21.1 (2023): 261.
