Editor: Nina
Scientists develop a sonoporation-assisted micelle delivery system that enhances the targeted delivery and distribution of drugs to glioblastoma tumors, offering potential improvements over conventional cancer treatment methods.
Key Preview
Research Question:
The study aims to improve tumor-specific drug delivery for glioblastoma treatment, focusing on increasing the precision and efficiency of micelle-based nanocarrier systems using sonoporation.
Research Design and Strategy:
The researchers employed an innovative strategy combining micelles with ultrasound-triggered sonoporation to improve the penetration and distribution of these carriers within glioblastoma tissues. This approach seeks to address the challenges of suboptimal drug delivery and tumor tissue penetration commonly encountered in traditional treatment methods.
Method:
The study used bi-modal imaging techniques, including positron emission tomography (PET) and optical imaging, to track the distribution of zirconium-89-labeled micelles in a subcutaneous glioblastoma mouse model. The efficacy of micelle delivery was further assessed with and without the assistance of microbubble-induced sonoporation.
Key Results:
The results demonstrated that sonoporation significantly enhanced the uniform distribution and retention of the micelles in the tumor tissue. PET imaging showed that the micelles accumulated efficiently in the tumor with sustained retention over 48-72 hours post-injection, and their clearance was predominantly via the hepato-biliary system. Sonoporation also improved the homogeneity of drug delivery within the tumor.
Significance of the Research:
This study showcases a promising strategy for overcoming the limitations of current drug delivery methods in cancer therapy, particularly for glioblastoma. The combination of sonoporation with micelle delivery has the potential to improve therapeutic outcomes by ensuring more efficient, precise, and sustained drug release within tumors, thus enhancing treatment effectiveness and minimizing side effects.
Introduction
Glioblastoma, one of the most aggressive and difficult-to-treat cancers, poses significant challenges in drug delivery due to the highly heterogeneous and dense nature of the tumor tissue. Traditional strategies for delivering chemotherapy to tumors often rely on the enhanced permeability and retention (EPR) effect, which allows nanocarriers to passively accumulate in tumors through leaky blood vessels. However, this approach suffers from issues such as poor drug diffusion and uneven distribution within the tumor, leading to suboptimal therapeutic effects.
Conventional methods often fail to ensure sufficient drug penetration, which results in limited treatment efficacy. The heterogeneous nature of the tumor environment further complicates the effective delivery of therapeutic agents to all areas of the tumor, particularly the deeper, more inaccessible regions.
The innovative approach presented in this study introduces sonoporation-assisted micelle delivery, a method that combines ultrasound and microbubbles to transiently open the tumor vasculature, thereby allowing better penetration and distribution of drug-loaded micelles throughout the tumor.
Research Team and Aim
The study was led by Estelle Porret and Stéphane Hoang, with contributions from researchers at Université Paris-Saclay, CEA, and the Czech Academy of Sciences. The paper, titled “Sonoporation-assisted micelle delivery in subcutaneous glioma-bearing mice evaluated by PET/fluorescent bi-modal imaging,” was published in Nanoscale in 2023.
The primary aim of the research was to evaluate the in vivo efficacy of sonoporation-assisted micelle delivery in a glioblastoma mouse model, particularly focusing on the enhancement of drug delivery and distribution in tumor tissues using ultrasound-assisted microbubble sonoporation.
Experimental Process
Primary Technique:
The primary technique used in this study is a combination of bi-modal imaging and sonoporation-assisted micelle delivery. This method integrates two powerful imaging techniques—positron emission tomography (PET) and optical fluorescence imaging—to track the distribution of micelles within a glioblastoma (U-87 MG) mouse model. Additionally, sonoporation, a non-invasive technique using ultrasound and microbubbles, was employed to enhance the delivery and penetration of the micelles into the tumor tissue.
Key Steps:
1.Micelle Preparation:
The researchers synthesized polymerized micelles by combining two amphiphilic molecules: diacetylene poly(ethylene glycol) (DA-PEG) and diacetylene deferoxamine (DA-DFO). These were mixed and self-assembled into micelles, which were then loaded with a near-infrared fluorophore (DiD) for optical imaging. For PET imaging, the micelles were radiolabeled with zirconium-89 ([89Zr]). The micelles were purified through size-exclusion chromatography, ensuring their proper size and stability for in vivo studies.
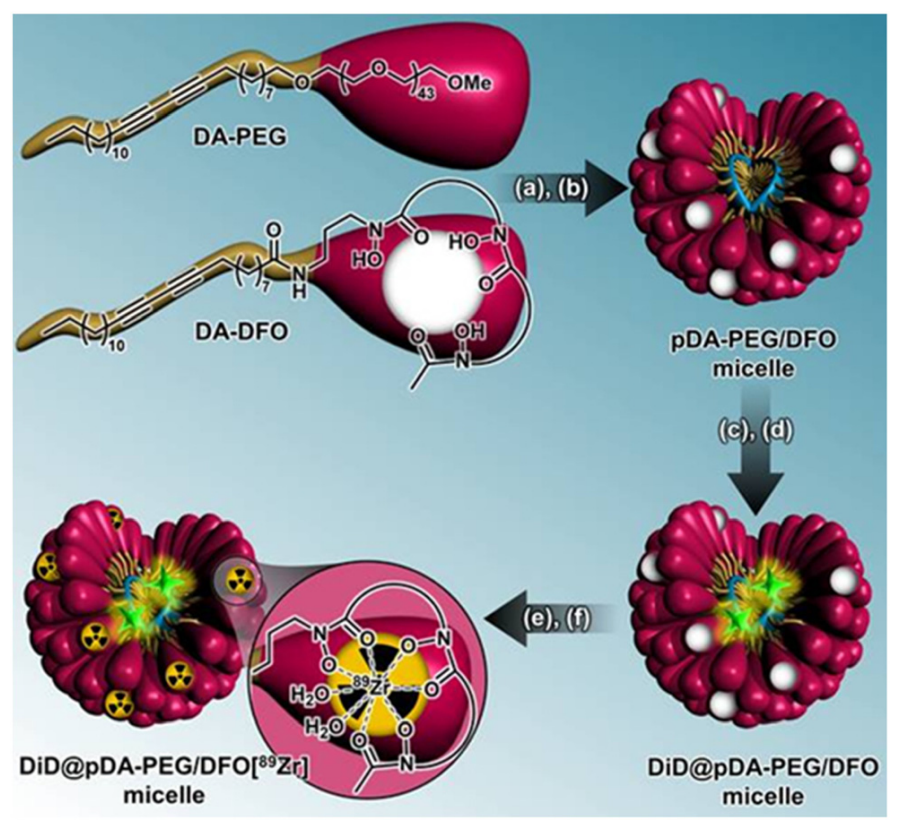
scheme 1. Micelle synthesis steps with radiolabeling strategy. Step 1: (a) formation of micelles in aqueous solution (>CMC, pH 12) and (b) polymerization by UV irradiation (254 nm). Step 2: (c) DiD encapsulation (λexc ∼ 645 nm, λem. ∼ 665 nm) and (d) pre-purification a PD-10 column at pH 7.8. Step 3: (e) radiolabeling of the micelles platform using 89 Zr and (f) post-purification via a PD-10 column and vivaspin.
2. Sonoporation Protocol:
Microbubbles were injected intravenously into the mice, followed by the application of ultrasound (US) to the tumors. This process uses acoustic waves to temporarily open the blood vessels within the tumor, enhancing the permeability of the tumor vasculature and allowing better diffusion and deeper penetration of the micelles into the tumor tissue. This is a critical step to improve micelle distribution across the tumor’s heterogenous structure.
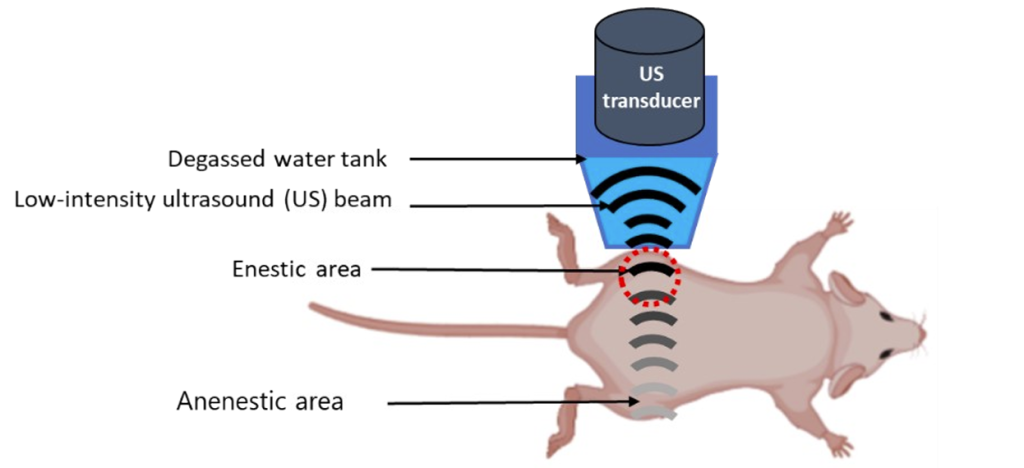
Figure 1. Experimental setup for Sonoporation procedure.
3. In Vivo Imaging and Micelle Tracking:
After administering the micelles with or without microbubbles and ultrasound exposure, the mice were imaged using both PET and optical fluorescence imaging. The PET scans provided detailed information on the pharmacokinetics and biodistribution of the micelles, while optical imaging allowed the visualization of the micelles’ location within the tumor using the DiD fluorophore. Imaging was conducted at multiple time points (e.g., 4, 24, 48, 72 hours post-injection) to track the micelles’ accumulation in the tumor over time.
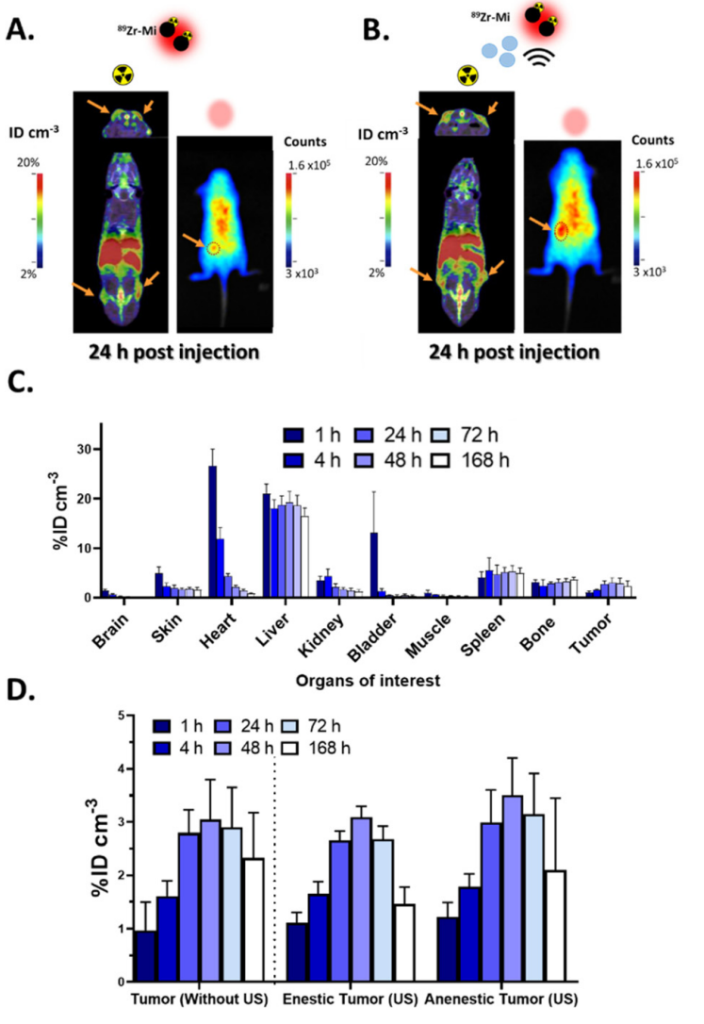
Figure 1. In vivo biodistribution of DiD@pDA–PEG/DFO[ 89 Zr] micelles in subcutaneous U87-MG tumor model. PET (coronal-upper and axiallower) and optical images obtained from mice
4. Ex Vivo Tissue Analysis:
Following the in vivo imaging, the mice were euthanized, and their tumors and key organs (such as the liver, spleen, and kidneys) were excised for ex vivo analysis. These tissue samples were analyzed using autoradiography and fluorescence microscopy to confirm the results obtained through PET and optical imaging. This validation process helped ensure that the imaging results accurately reflected the micelles’ distribution and retention.
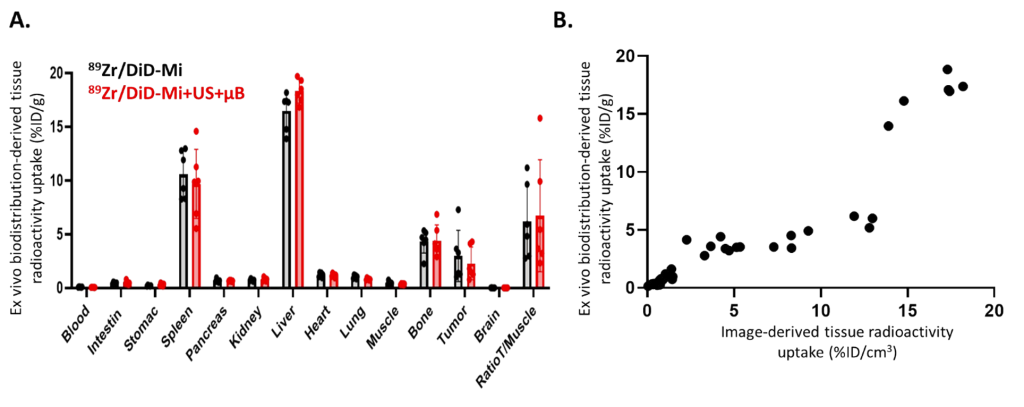
Figure 2. Ex vivo analyses obtained one week after DiD@pDA-PEG/DFO[89Zr] micelle injection in mice bearing subcutaneous U87-MG tumors.
Data Collection and Analysis:
The data were collected through longitudinal imaging with PET and fluorescence techniques, providing both qualitative and quantitative data. The PET scans were used to track the total uptake and distribution of the radiolabeled micelles over time, while fluorescence imaging allowed for high-resolution visualization of micelle location within the tumor. Statistical analysis was employed to compare the micelle accumulation in tumors with and without ultrasound treatment, focusing on differences in the distribution uniformity and retention duration. Results were analyzed for significant differences using appropriate statistical tests, such as ANOVA.
Novel Aspects and Advantages:
The novel aspect of this study lies in the integration of sonoporation with micelle drug delivery, which has not been widely explored. Traditional nano-delivery systems often suffer from uneven distribution and poor tumor penetration due to the dense and heterogeneous nature of tumors. The use of sonoporation allows for temporary disruption of the tumor vasculature, significantly improving the diffusion and homogeneity of drug-loaded micelles within the tumor. This technique enhances the precision and effectiveness of drug delivery, overcoming some of the key limitations of conventional methods that rely solely on passive accumulation via the enhanced permeability and retention (EPR) effect. By combining bi-modal imaging for real-time tracking and ultrasound for enhanced delivery, this study provides a more efficient and targeted drug delivery system compared to traditional nano-carriers.
Conclusion
The successful development of the drug delivery system was achieved by combining micelle-based nanocarriers with sonoporation, which significantly enhanced the targeted and uniform delivery of drugs to glioblastoma tumors. The study’s findings highlight the potential of this innovative method to overcome the challenges of traditional drug delivery systems, particularly in delivering effective treatments to dense, heterogeneous tumors like glioblastoma. By utilizing ultrasound and microbubbles to facilitate better micelle distribution, this approach could pave the way for more efficient and precise cancer therapies.
Reference
Porret, Estelle, et al. “Sonoporation-Assisted Micelle Delivery in Subcutaneous Glioma-Bearing Mice Evaluated by PET/Fluorescent Bi-Modal Imaging.” Nanoscale, vol. 15, no. 37, 2023, pp. 12574-12585.
