Editor: Tiffany
Researchers have discovered that a molecule called hsa_circ_0136666 plays a crucial role in helping gastric cancer cells grow and avoid detection by the immune system, offering new insights into potential treatments.
Key Highlights
- Research Question:
How does hsa_circ_0136666 influence gastric cancer progression and immune escape mechanisms? - Research Difficulties:
Identifying the specific roles of circular RNAs in cancer biology, particularly regarding immune responses. - Key Findings:
hsa_circ_0136666 promotes gastric cancer cell proliferation and immune evasion by sponging miR-375 and enhancing PD-L1 phosphorylation, leading to tumor immune escape. - Innovative Aspects:
The study identifies hsa_circ_0136666 as a novel target for enhancing anti-PD-L1 therapy effectiveness. - Importance of the Study:
This research provides new insights into gastric cancer mechanisms and suggests therapeutic strategies that could improve patient outcomes.
Gastric Cancer: A Major Health Challenge and Need for Innovative Therapies
Gastric cancer, a malignancy originating in the stomach, poses a significant global health challenge, claiming over 700,000 lives annually and ranking as the third leading cause of cancer-related mortality worldwide. Its prevalence is notably high in East Asia, where it stands as the most common cancer, influenced by factors like diet and Helicobacter pylori infection. Symptoms—including abdominal pain, unexplained weight loss, and difficulty swallowing—typically emerge in advanced stages, often resembling benign gastrointestinal issues, which delays diagnosis and worsens outcomes. Current treatments, such as chemotherapy and targeted therapies like trastuzumab for HER2-positive cases, face substantial hurdles. These include limited efficacy due to drug resistance, non-specific effects on healthy cells, and the tumor’s sophisticated ability to evade immune detection. In response, research has increasingly explored circular RNAs (circRNAs), a class of non-coding RNAs known to regulate gene expression. These molecules are gaining attention as potential biomarkers and therapeutic targets in oncology. This study investigates hsa_circ_0136666, a specific circRNA, examining its role in driving gastric cancer progression and facilitating immune escape, offering new insights into this deadly disease.
Investigating the Role of hsa_circ_0136666 in Gastric Cancer Progression and Immune Evasion
Gastric cancer remains a formidable challenge in modern medicine, largely because existing treatments struggle to overcome the cancer’s ability to resist drugs and evade the body’s immune defenses. To address these persistent difficulties, researchers are working to unravel the complex molecular mechanisms driving the disease. This study, led by Zhenyan Miao and a team of scientists from esteemed institutions, including the School of Biotechnology at Jiangsu University, set out to explore one such mechanism in detail. Specifically, their aim was to investigate the role of a circular RNA molecule known as hsa_circ_0136666 and how it influences gastric cancer progression.
The team zeroed in on the intricate interactions between hsa_circ_0136666, a microRNA called miR-375, and a protein named PRKDC. Their research sought to determine how this molecular trio collaborates to fuel cancer cell growth while simultaneously helping tumors dodge immune system attacks. By dissecting these relationships, the study aimed to shed light on why current therapies often fail and to identify potential weak points in the cancer’s defenses that could be targeted with new treatments. Published in the prestigious journal Molecular Cancer in 2023, their findings represent a timely and critical step forward in the quest to develop more effective strategies for combating gastric cancer, offering hope for improved patient outcomes in the future.
Experimental Approach: Unraveling the Function and Mechanism of hsa_circ_0136666
(1) Outline of the Experimental Process
- Sample Collection: Acquire gastric cancer tissues and cell lines for analysis.
- CircRNA Identification: Use Gene Expression Omnibus (GEO) repository to screen for differentially expressed circRNAs.
- Validation of hsa_circ_0136666: Confirm the existence of hsa_circ_0136666 through Sanger sequencing and RNase R digestion.
- Expression Analysis: Perform quantitative reverse transcription-PCR (QRT-PCR) to assess expression levels of hsa_circ_0136666 and PRKDC.
- Cell Proliferation Assays: Conduct CCK-8 assays to evaluate the effects of hsa_circ_0136666 on gastric cancer cell proliferation.
- Immune Response Assessment: Analyze immune checkpoint protein expression and immune cell infiltration in co-culture and mouse models.
- Mechanistic Studies: Execute RNA immunoprecipitation (RIP) and dual luciferase reporter assays to investigate the interaction between hsa_circ_0136666 and miR-375.
- In Vivo Studies: Establish murine models to evaluate tumor growth and immune response in the presence of hsa_circ_0136666.
- Therapeutic Evaluation: Test the efficacy of siRNA-LNP in combination with anti-PD-L1 therapy in vivo.
(2) Key Experiments
1. Validation of hsa_circ_0136666
- Procedure: Sanger sequencing and RNase R digestion were performed to confirm the presence and circular structure of hsa_circ_0136666 in gastric cancer cells.
- Result: The sequencing confirmed the back-splice site of hsa_circ_0136666, and RNase R digestion showed that hsa_circ_0136666 was resistant to degradation, indicating its stability.
- New Finding: This experiment established hsa_circ_0136666 as a stable circular RNA in gastric cancer cells, suggesting its potential significance in tumor biology.
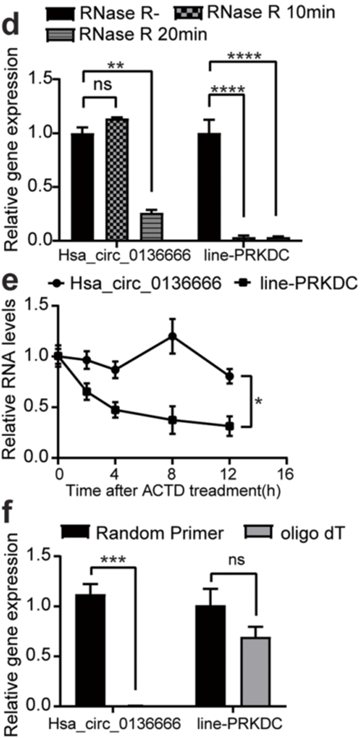
Figure 1. (d) Hsa_circ_0136666 and linear-PRKDC gene expression abundance were detected under RNase digestion, n=3. (e) The natural degradation rate of hsa_circ_0136666 and linear-PRKDC gene was detected under Actinomycin D treatment, n=3. (f) Hsa_circ_0136666 and linear PRKDC genes were amplifed using oligo dT and random primers, n=3.
2. Impact on Cell Proliferation
- Procedure: CCK-8 assays were conducted on gastric cancer cells with overexpressed or knocked down hsa_circ_0136666 to measure cell viability and proliferation rates.
- Result: Overexpression of hsa_circ_0136666 led to increased cell proliferation, while knockdown resulted in significantly decreased growth.
- New Finding: This demonstrated that hsa_circ_0136666 plays a crucial role in promoting gastric cancer cell proliferation.
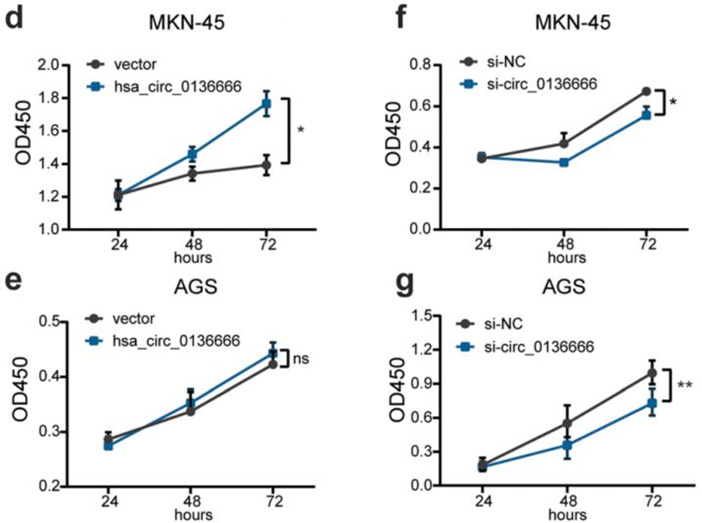
Figure 2. (d) Overexpression of hsa_circ_0136666 promoted cell proliferation in MKN-45 cells. (e) Overexpression of hsa_circ_0136666 promoted cell proliferation in AGS cells. (f) Knockdown of hsa_circ_0136666 inhibited cell proliferation in MKN-45 cells. (g) Knockdown of hsa_circ_0136666 inhibited cell proliferation in AGS cells.
3. Immune Response Assessment
- Procedure: Co-culture experiments with CD8+ T cells were performed to analyze the effects of hsa_circ_0136666 on immune evasion in gastric cancer cells.
- Result: Gastric cancer cells overexpressing hsa_circ_0136666 displayed a greater survival rate when co-cultured with CD8+ T cells compared to control cells.
- New Finding: This indicated that hsa_circ_0136666 contributes to immune escape mechanisms by enhancing tumor cell resistance to immune-mediated destruction.
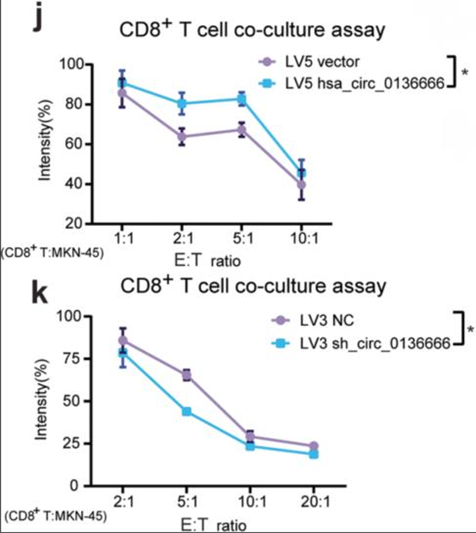
Figure 3. (j) Image of survival rate of overexpressing hsa_circ_0136666 tumor cells co-cultured with CD8+ T cells, n=6. (k) Image of survival rate of knockdown hsa_circ_0136666 tumor cells co-cultured with CD8+ T cells, n=6.
4. Mechanistic Studies
- Procedure: RNA immunoprecipitation (RIP) and dual luciferase reporter assays were used to analyze the interaction between hsa_circ_0136666 and miR-375, confirming the sponge effect.
- Result: The assays confirmed that hsa_circ_0136666 binds to miR-375, preventing its inhibitory effects on target genes like PRKDC.
- New Finding: This established the role of hsa_circ_0136666 as a miR-375 sponge, elucidating its mechanism in promoting gastric cancer progression.
5. In Vivo Studies
- Procedure: Murine models were established by transplanting gastric cancer cells overexpressing hsa_circ_0136666 to observe tumor growth and immune microenvironment changes.
- Result: Mice with tumors overexpressing hsa_circ_0136666 exhibited faster tumor growth and altered immune cell infiltration compared to controls.
- New Finding: This confirmed that hsa_circ_0136666 enhances tumor progression and modifies the immune landscape, supporting its role in immune evasion in vivo.
Key Findings: Insights into hsa_circ_0136666’s Oncogenic Role and Therapeutic Potential
This study highlights the significant role of hsa_circ_0136666 as an oncogenic circular RNA in gastric cancer, specifically functioning as a sponge for miR-375. This mechanism inhibits the tumor-suppressive effects of miR-375, leading to the upregulation of PRKDC and, consequently, the phosphorylation and stabilization of PD-L1. This research provides innovative insights into the interplay between circular RNAs and immune checkpoint regulation, suggesting that hsa_circ_0136666 could serve as a novel therapeutic target to enhance the efficacy of anti-PD-L1 therapies in gastric cancer.
Despite these advancements, the study has certain limitations. The focus on hsa_circ_0136666, while significant, does not encompass the broader landscape of differentially expressed circRNAs that may also contribute to gastric cancer progression. Additionally, the investigation primarily emphasizes the miR-375 sponge function of hsa_circ_0136666, leaving other potential roles, such as interactions with RNA-binding proteins, unexplored. Furthermore, the effectiveness of the siRNA-LNP treatment was not as pronounced as anticipated, indicating that combining this approach with additional therapeutic strategies could improve outcomes. Future research should aim to investigate the multifaceted roles of hsa_circ_0136666 and other circRNAs in gastric cancer, validate the findings in clinical settings, and explore combination therapies to fully harness the potential of targeting this circular RNA.
Reference:
Miao, Zhenyan, et al. “Hsa_circ_0136666 stimulates gastric cancer progression and tumor immune escape by regulating the miR-375/PRKDC Axis and PD-L1 phosphorylation.” Molecular Cancer 22.1 (2023): 205.
