Editor: Tiffany
Researchers have developed advanced biomimetic nanorobots that significantly enhance drug delivery and penetration in treatment-resistant triple-negative breast cancer.
Key Highlights
- Research Question:
How can nanocarrier systems be improved to enhance drug delivery efficiency for triple-negative breast cancer (TNBC) characterized by dense stromal barriers? - Research Difficulties:
Current nanocarrier systems struggle with limited tumor penetration and low cellular internalization efficiency, particularly in TNBC with a stiff tumor microenvironment. - Key Findings:
The newly designed biomimetic nanorobots demonstrate effective remodeling of tumor stroma, enabling deep penetration and improving drug delivery, significantly suppressing tumor growth in various models. - Innovative Aspects:
The nanorobots utilize a site-selective superassembly strategy that combines active movement, spiky heads for enhanced cellular uptake, and a hollow tail for targeted drug delivery. - Importance of the Study:
This research addresses major limitations in traditional cancer therapies and opens pathways for more effective treatments for TNBC and potentially other cancers with dense stroma.
Understanding Triple-Negative Breast Cancer
Triple-negative breast cancer (TNBC) is a particularly aggressive subtype of breast cancer that poses a significant challenge due to its lack of targeted therapies. Approximately 15-20% of breast cancer cases are classified as TNBC, which is characterized by the absence of estrogen receptors, progesterone receptors, and HER2 expression. This subtype is associated with a higher risk of metastasis, particularly to the bones, and is often resistant to conventional chemotherapy. The dense tumor microenvironment, composed of fibrotic tissue and a viscous extracellular matrix, acts as a formidable barrier to effective drug delivery. Consequently, traditional treatment options frequently yield suboptimal outcomes for patients, underscoring the urgent need for innovative therapeutic strategies that enhance drug penetration and efficacy in TNBC.
Research Aim & Objectives
This study aims to develop and evaluate a novel biomimetic nanorobot system designed to improve drug delivery efficiency specifically for triple-negative breast cancer (TNBC). The primary objective is to address the significant barriers presented by the dense tumor microenvironment, which hampers the penetration and internalization of therapeutic agents. To achieve this aim, the research focuses on the following key objectives:
- Design and Synthesis: Create biomimetic nanorobots with a unique head and hollow tail structure that can actively navigate through the tumor stroma, utilizing advanced materials and engineering techniques to optimize their physical properties.
- Mechanistic Understanding: Investigate the mechanisms by which these nanorobots remodel the tumor microenvironment, enhancing their ability to penetrate deeply into the tumor tissue and facilitate drug delivery.
- Drug Delivery Efficiency: Assess the efficacy of the nanorobots in delivering chemotherapeutic agents, such as doxorubicin, and evaluate their impact on tumor growth suppression in various in vitro and in vivo models of TNBC.
- Comparative Analysis: Compare the performance of these innovative nanorobots with traditional nanocarrier systems, focusing on cellular uptake, transvascular extravasation, and overall therapeutic outcomes.
By achieving these objectives, the study aims to provide a new strategy for overcoming the limitations of current TNBC treatments and improve patient outcomes through enhanced drug delivery mechanisms.
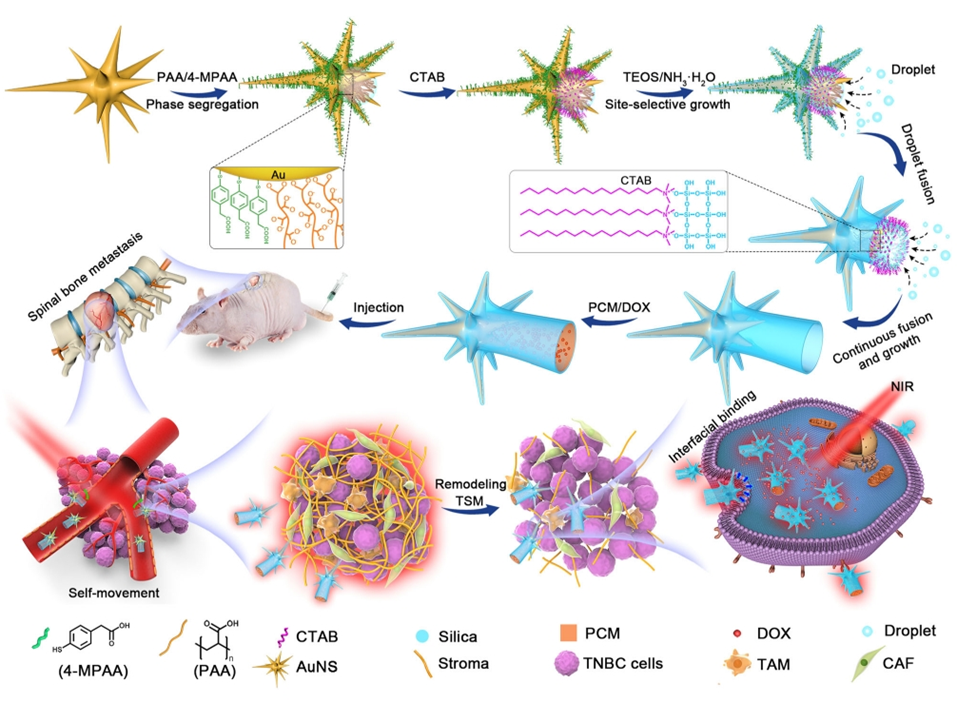
Figure 1. Design of the superassembled nanorobot for the treatment of TNBC spinal bone metastasis with stiff stroma.
Experimental Design and Key Findings
(1) Experimental Process Outline
- Synthesis of Gold Nanostars (AuNSs): Prepare AuNSs through a reduction process using gold chloride and silver nitrate.
- Design and Assembly of Biomimetic Nanorobots: Employ a site-selective superassembly strategy to create asymmetric urchin head/hollow tail nanostructures (UHHTNs).
- Characterization of Nanorobots: Utilize transmission electron microscopy (TEM) and scanning electron microscopy (SEM) for morphological assessment.
- In Vitro Studies: Evaluate cellular uptake and internalization efficiency using various cancer cell lines.
- In Vivo Studies: Establish TNBC mouse models and administer UHHTN drug delivery systems.
- Evaluation of Therapeutic Efficacy: Monitor tumor growth suppression and assess bioluminescence imaging for tumor tracking.
- Histological Analysis: Perform histological examinations and immunofluorescence staining to assess tumor microenvironment remodeling.
(2) Key Experiments
1. Synthesis and Characterization of UHHTNs
- Procedure: Gold nanostars were synthesized and modified with a silica shell using a combination of 4-mercaptophenylacetic acid and poly(acrylic acid) to create asymmetric nanostructures. The morphology and structural integrity were confirmed using TEM and SEM imaging.
- Result: Successfully synthesized UHHTNs displayed a distinctive spiky head and an open hollow tail, with precise control over their dimensions.
- New Finding: The site-selective superassembly strategy allowed for fine-tuning of the surface morphology, enhancing the properties necessary for improved cellular interaction and drug delivery.
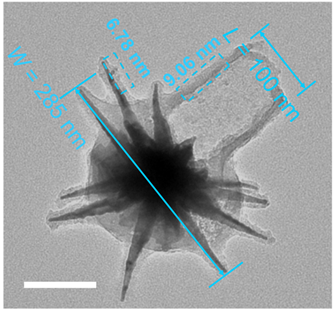
Figure 2. Magnified TEM image showing an individual UHHTN with sharp surface spikes and an opening of ~100 nm at the end of the hollow tail (Lopening), a head width (Whead) of ~285 nm (indicated by blue line).
2. In Vitro Cellular Uptake and Internalization
- Procedure: MDA-MB-231 cancer cells were treated with both spiky and smooth nanostructures, with and without near-infrared (NIR) laser irradiation, to analyze cellular uptake via confocal microscopy.
- Result: The spiky nanorobots demonstrated significantly higher internalization efficiency compared to smooth counterparts, particularly under NIR irradiation.
- New Finding: The unique spiky morphology, combined with NIR activation, markedly enhances cellular uptake, indicating the potential for more effective drug delivery in clinical applications.
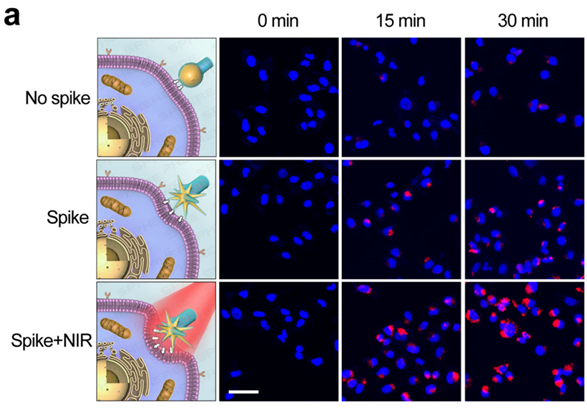
Figure 3. Fluorescence images of MDA-MB-231 cells treated with nanoparticles without nanospikes, spiky nanoparticles and spiky nanorobots after 0, 15 and 30 min of intervention.
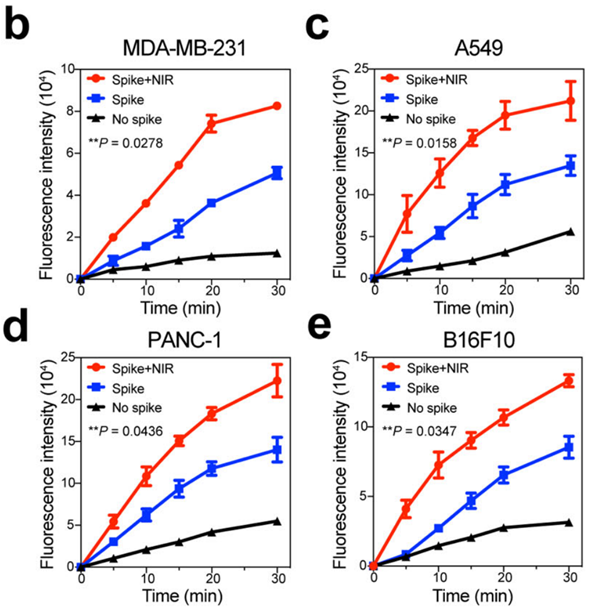
Figure 4. Internalized fluorescence density of nanoparticles without nanospikes, spiky nanoparticles and spiky nanorobots in (b) MDA-MB-231, © A549, (d) PANC-1 and (e) B16F10 tumor cells, respectively.
3. In Vivo Efficacy in TNBC Mouse Models
- Procedure: TNBC mouse models were established by injecting MDA-MB-231 cells into the spinal area. Post-injection, mice were treated with UHHTN/DOX nanorobots and subjected to NIR irradiation.
- Result: The UHHTN/DOX group showed a significant reduction in tumor growth, with bioluminescence imaging confirming a decrease in tumor volume compared to control groups.
- New Finding: The combination of photothermal therapy and targeted drug delivery via UHHTNs resulted in enhanced therapeutic efficacy, illustrating the nanorobots’ potential in overcoming treatment resistance in TNBC.
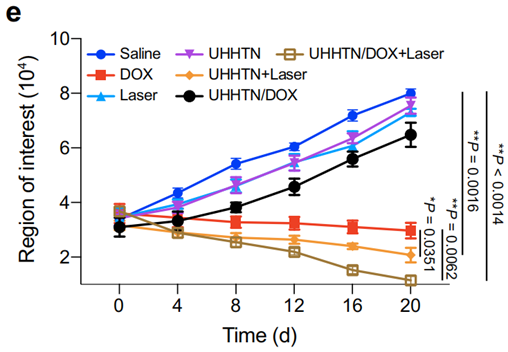
Figure 5. Fluorescence analysis of regions of interest for TNBC spinal tumors on bioluminescence imaging at multiple time points after receiving 20 days.
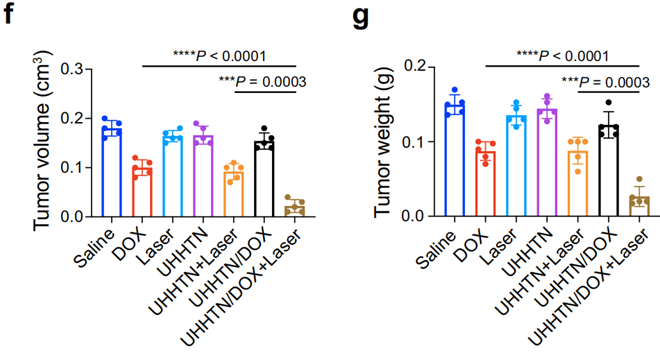
Figure 6. (f) Tumor weight and (g) volume in nude mice with TNBC spinal metastasis after different 20 days treatments at a DOX dosage of 5 mg kg−1 once every four days.
Implications of Nanorobots in Cancer Treatment
This study introduces a novel approach to improve drug delivery in triple-negative breast cancer (TNBC) through the development of biomimetic nanorobots featuring an asymmetric urchin head/hollow tail structure. These nanorobots effectively integrate multiple functionalities, including enhanced tumor penetration, active cellular internalization, and controlled drug release. The use of a site-selective superassembly strategy allows for precise control over their morphology, which is essential for improving therapeutic efficacy.
The significance of this research lies in its ability to address the barriers posed by the dense tumor microenvironment, which limits the effectiveness of conventional chemotherapy. The findings demonstrate that the nanorobots can remodel tumor stroma and facilitate better access to cancer cells, leading to significant tumor growth suppression in various in vivo models. By combining photothermal therapy with targeted drug delivery, this approach enhances treatment outcomes for patients with treatment-resistant cancers.
Overall, this study highlights the potential of biomimetic nanorobots as a promising tool for cancer therapy and suggests pathways for future research to optimize their design and functionality for clinical applications. The integration of nanotechnology with cancer treatment strategies may contribute to improved outcomes for aggressive cancer types like TNBC
Reference:
Yan, Miao, et al. “Site-selective superassembly of biomimetic nanorobots enabling deep penetration into tumor with stiff stroma.” Nature Communications 14.1 (2023): 4628.
