Editor: Tiffany
A recent study has developed a novel nanoplatform that improves the efficacy of phototherapy for cervical cancer, combining targeted drug delivery with advanced imaging techniques to enhance treatment outcomes.
Key Highlights
- Research Question:
How can a nanoplatform be designed to improve treatment efficacy for cervical cancer through targeted therapy and imaging? - Research Difficulties:
The challenges included enhancing the stability and tumor-specific targeting of indocyanine green (ICG), a photosensitizer used in phototherapy, while minimizing side effects. - Key Findings:
The newly designed M-HMnO2@ICG nanoparticles demonstrated superior antitumor effects and biocompatibility, effectively targeting tumor cells and enhancing photodynamic therapy outcomes. - Innovative Aspects:
The study introduces a novel, tumor microenvironment-responsive delivery system that utilizes HeLa cell membrane coatings to enhance targeting and immune evasion. - Importance of the Study:
This research provides a promising new strategy for cervical cancer treatment, potentially leading to better patient outcomes and fewer side effects compared to conventional therapies.
Cervical Cancer: Current Treatment Challenges and Nanotechnology Advances
Cervical cancer is one of the most prevalent gynecological cancers globally, with the World Health Organization estimating over 300,000 deaths annually. This disease often presents with symptoms such as abnormal bleeding, pelvic pain, and discomfort during intercourse, significantly impacting the quality of life for affected individuals. Traditional treatment options, including surgery, chemotherapy, and radiation, can have adverse effects, particularly in younger patients who wish to preserve their fertility. Moreover, the limitations of standard therapies underscore the urgent need for innovative, targeted approaches that minimize side effects while enhancing efficacy. Recent advancements in nanotechnology and phototherapy present promising avenues for developing novel treatment modalities that could revolutionize cervical cancer management by providing precise targeting and improved therapeutic outcomes.
Research Aim & Objectives
The research team, led by Ying Wang and including collaborators from various institutes, published their findings in the International Journal of Nanomedicine. The primary aim of this study was to develop a novel nanoplatform that enhances the efficacy of phototherapy for cervical cancer through improved targeted drug delivery and multimodal imaging capabilities. Specifically, the objectives included:
- Nanoparticle Design: To construct hollow mesoporous manganese dioxide (HMnO2) nanoparticles that can efficiently load the photosensitizer indocyanine green (ICG) while ensuring stability and tumor specificity.
- Targeted Delivery: To modify the nanoparticles with polyethyleneimine and HeLa cell membrane coatings, facilitating targeted accumulation in cervical cancer cells and evasion of the immune system.
- Therapeutic Enhancement: To investigate the synergistic effects of combined photothermal therapy (PTT), photodynamic therapy (PDT), and chemodynamic therapy (CDT) facilitated by the nanoplatform, aiming to maximize antitumor efficacy.
- Imaging Capabilities: To incorporate multimodal imaging functions, enabling real-time monitoring of treatment response and therapeutic efficacy through fluorescence and magnetic resonance imaging.
By achieving these objectives, the study aimed to establish a comprehensive and innovative treatment strategy for cervical cancer that addresses the limitations of current therapies and improves patient outcomes.
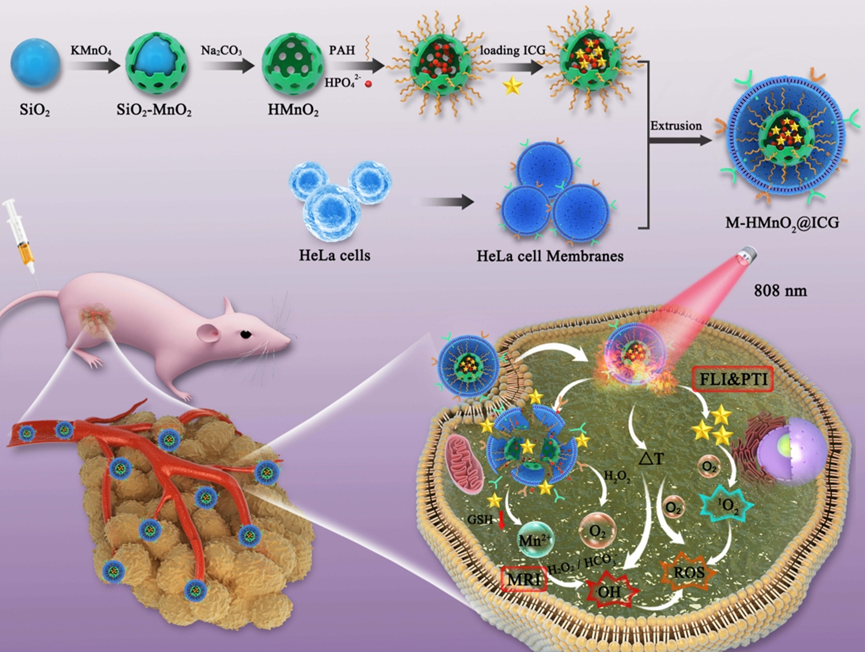
Figure 1. Schematic diagram of M-HMnO2@ICG preparation and multimodal imaging-guided PTT/PDT/CDT.
Nanoparticle Synthesis, Characterization, and Functional Assessment
(1) Experimental Process Outline
- Synthesis of hollow mesoporous manganese dioxide nanoparticles (HMnO2).
- Modification of HMnO2 with poly(allylamine hydrochloride) (PAH) for charge reversal.
- Loading of indocyanine green (ICG) onto HMnO2 via counterion aggregation using hydrogen phosphate ions.
- Coating of nanoparticles with HeLa cell membrane fragments to enhance targeting and immune evasion.
- Evaluation of photothermal properties of M-HMnO2@ICG nanoparticles.
- Assessment of oxygen generation efficiency in tumor microenvironment conditions.
- Investigation of photodynamic properties using 9,10-anthracenediyl-bis (methylene) dimalonic acid (ABDA).
- In vitro cytotoxicity testing using CCK8 assay on HeLa cells.
- In vivo antitumor efficacy evaluation using a mouse model of cervical cancer.
(2) Key Experiments Introduction:
1. Synthesis of HMnO2 Nanoparticles
Procedure: The synthesis of HMnO2 nanoparticles involved an oxidation-reduction reaction using KMnO4 and SiO2, followed by etching with sodium carbonate to create hollow structures. The nanoparticles were then modified with PAH to enhance their stability and loading capacity for ICG.
Result: The resulting HMnO2 nanoparticles exhibited a unique hollow structure with a high specific surface area, facilitating efficient drug loading. The encapsulation efficiency of ICG was significantly improved to 95.14% when using the modified HMnO2/PAH/HPO4²⁻ carrier system.
New Finding: This experiment demonstrated that the combination of hollow mesoporous structures and surface modifications greatly enhances the loading capacity and stability of ICG, setting the stage for improved therapeutic delivery.
2. Evaluation of Photothermal Properties
Procedure: The photothermal properties of M-HMnO2@ICG were assessed by irradiating aqueous solutions of the nanoparticles with a near-infrared (NIR) laser and monitoring the temperature changes over time.
Result: The nanoparticles demonstrated a significant temperature increase, achieving a maximum temperature of 51.3°C under 1.35 W/cm² laser irradiation, indicating effective photothermal conversion.
New Finding: This experiment confirmed that the M-HMnO2@ICG nanoparticles possess excellent photothermal properties, which can be harnessed for effective tumor ablation when used in conjunction with NIR laser therapy.
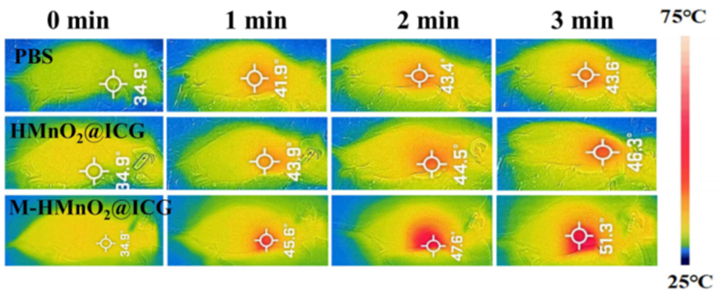
Figure 2. Photothermograms of HeLa tumor-bearing mice in different treatment groups under laser irradiation (808 nm, 1.35 W/cm2).
3. Assessment of Oxygen Generation Efficiency
Procedure: Oxygen generation capacity was evaluated using the RDPP probe in a reaction system containing M-HMnO2@ICG and hydrogen peroxide under acidic conditions, simulating the tumor microenvironment.
Result: The fluorescence intensity of RDPP decreased significantly in the presence of M-HMnO2@ICG, indicating effective O2 production, particularly in acidic conditions.
New Finding: This experiment revealed that the nanoparticles can self-generate oxygen in the tumor microenvironment, enhancing the efficacy of photodynamic therapy by providing a substrate for ICG to generate reactive oxygen species (ROS).
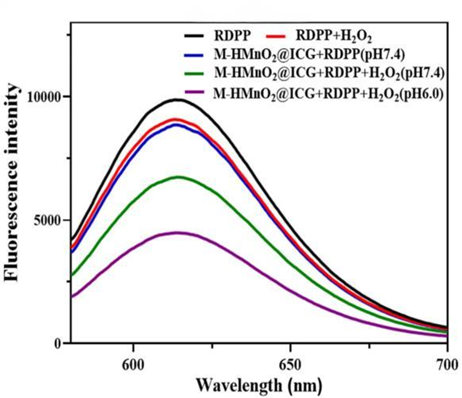
Figure 3. Fluorescence (RDPP probe) under different conditions reflecting M-HMnO2@ICG O2 generation.
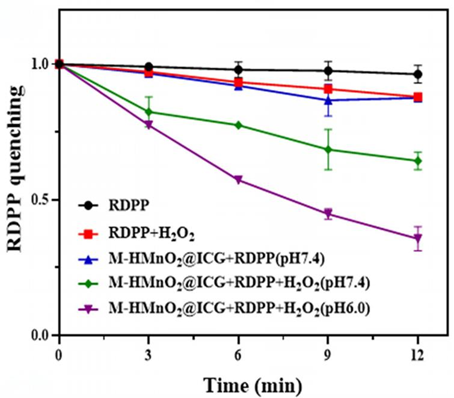
Figure 4. O2 production over time.
4. In Vitro Cytotoxicity Testing
Procedure: The in vitro cytotoxicity of M-HMnO2@ICG was assessed using the CCK8 assay on HeLa cells, comparing cell viability across different treatment groups, including control, ICG alone, and M-HMnO2@ICG with NIR irradiation.
Result: M-HMnO2@ICG exhibited a concentration-dependent reduction in cell viability, with an IC50 value of 34.7 µg/mL when exposed to NIR laser irradiation, significantly lower than the other treatment groups.
New Finding: This experiment underscored the synergistic antitumor effects of combined PTT/PDT/CDT facilitated by the M-HMnO2@ICG nanoparticles, highlighting their potential as a powerful therapeutic agent against cervical cancer.
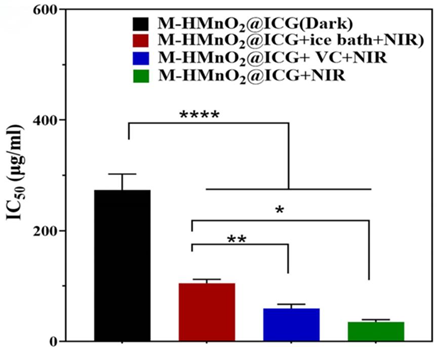
Figure 5. IC50s, calculated using the data illustrated in panel B.
Integrated Therapeutic Approach: Efficacy and Mechanistic Insights
In this study, a novel nano-biomimetic delivery system was designed, utilizing hollow mesoporous manganese dioxide (HMnO2) to enhance the therapeutic efficacy of indocyanine green (ICG) for cervical cancer treatment. The innovative approach involved the responsive degradation of HMnO2 in the tumor microenvironment, which facilitated the release of ICG while simultaneously generating oxygen and consuming glutathione to amplify oxidative stress. This dual mechanism not only improved the effectiveness of photodynamic therapy (PDT) but also allowed for the integration of photothermal therapy (PTT) and chemodynamic therapy (CDT), creating a synergistic treatment modality.
The significance of this research lies in its potential to overcome the limitations of traditional cervical cancer therapies, which often involve significant side effects and limited efficacy. By incorporating tumor cell membrane coatings, the nanoparticles achieved targeted delivery, enhancing accumulation in cancerous tissues while evading the immune system. This approach not only improves patient outcomes with fewer adverse effects but also sets a new precedent for the development of multimodal, tumor microenvironment-responsive nanoplatforms in cancer therapy. Overall, the findings of this study provide a promising framework for future advancements in targeted cancer treatments, highlighting the potential of nanotechnology in enhancing the effectiveness of existing therapeutic strategies.
Reference:
Wang, Ying, et al. “Tumor cell-targeting and tumor microenvironment–responsive nanoplatforms for the multimodal imaging-guided photodynamic/photothermal/chemodynamic treatment of cervical cancer.” International journal of nanomedicine (2024): 5837-5858.
