Editor: Sarah
Ovarian cancer remains one of the most challenging and lethal cancers to treat, primarily due to its late-stage diagnosis and resistance to conventional therapies. A recent study has introduced a novel strategy for improving the treatment of ovarian cancer by encapsulating dihydroartemisinin (DHA), a compound derived from the herb Artemisia annua, within a metal-organic framework (MOF). This approach, known as ZIF-DHA nanoparticles, enhances the bioavailability of DHA, increasing its anticancer effectiveness. The study focuses on overcoming the limitations of DHA’s solubility and bioavailability, aiming to offer a more targeted and effective treatment option for ovarian cancer patients.
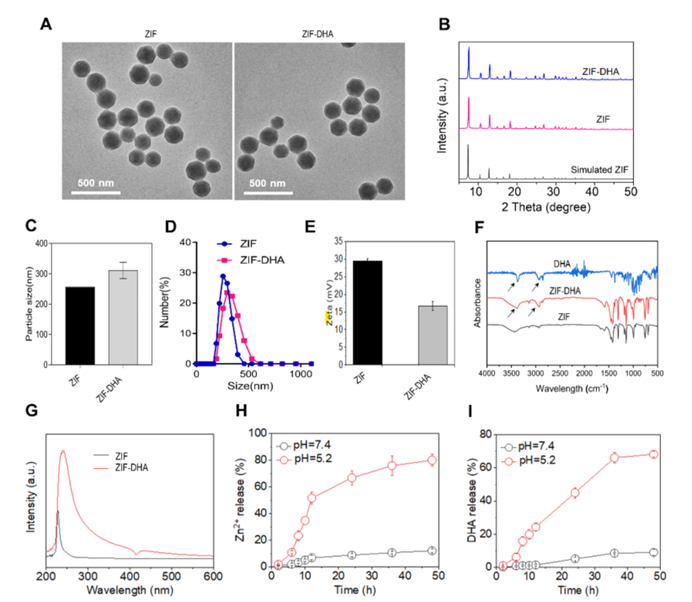
Figure 1: Characterization of ZIF-DHA nanoparticles.
Overcoming Limitations of DHA
Dihydroartemisinin, though promising as an anticancer agent, has faced significant challenges in clinical applications due to its poor solubility and low bioavailability. By encapsulating DHA within zeolitic imidazolate framework-8 (ZIF-8), a type of MOF, the researchers were able to create ZIF-DHA nanoparticles. These nanoparticles exhibited enhanced anticancer activity compared to free DHA. In ovarian cancer cell lines, ZIF-DHA effectively suppressed the production of reactive oxygen species (ROS), which are known to drive cancer cell proliferation. Additionally, ZIF-DHA induced apoptotic cell death. The study also identified that ROMO1, a mitochondrial protein, plays a crucial role in the therapeutic action of ZIF-DHA. Overexpression of ROMO1 in cancer cells reversed the effects of ZIF-DHA, shedding light on the molecular mechanisms involved.
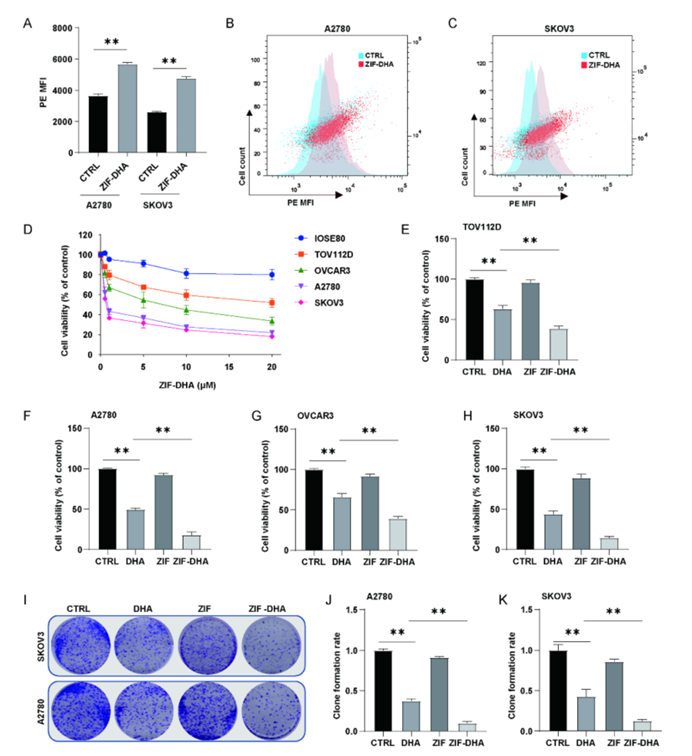
Figure 2: ZIF-DHA Suppresses Cell Growth in Ovarian Cancer Cells.
Methodology and Results
To validate the effectiveness of ZIF-DHA nanoparticles, the researchers employed several advanced techniques. Mass spectrometry (4D-FastDIA), molecular docking, and flow cytometry were used to analyze ROS levels and cell apoptosis. Both in vitro and in vivo models demonstrated that ZIF-DHA nanoparticles significantly inhibited tumor growth in ovarian cancer xenograft models. These results offer a deeper understanding of how ZIF-DHA interacts with ROMO1 and ROS production at the molecular level, supporting the robust methodology of the study.
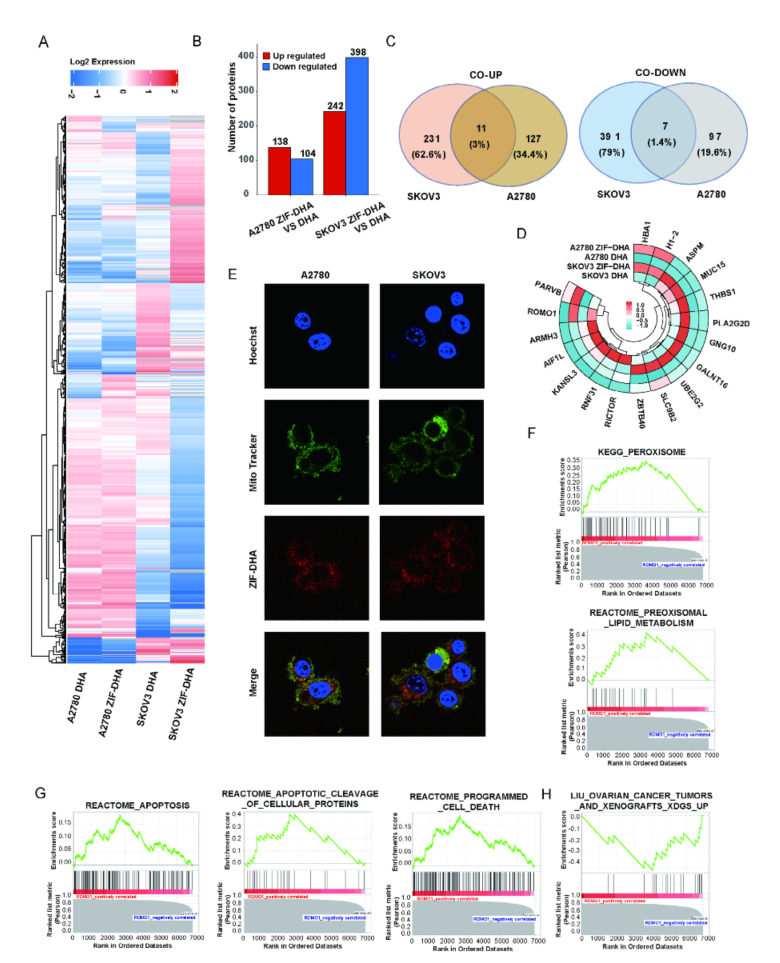
Figure 3: Differentially Expressed Proteins in Response to ZIF-DHA.
Contributions and Key Findings
- Enhanced Anticancer Activity: ZIF-DHA nanoparticles showed superior therapeutic activity compared to free DHA in ovarian cancer cell lines, notably reducing tumor growth and promoting apoptosis.
- ROMO1 as a Key Target: The study revealed that ROMO1, a mitochondrial protein, plays a critical role in the effects of ZIF-DHA. Suppression of ROMO1 expression led to a significant reduction in ROS production and increased cell apoptosis.
- Molecular Mechanisms: Through advanced mass spectrometry and molecular docking analysis, the researchers confirmed that ZIF-DHA targets ROMO1 to regulate oxidative stress. The nanoparticles were found to induce ROS-mediated apoptosis in ovarian cancer cells by disrupting the function of ROMO1.
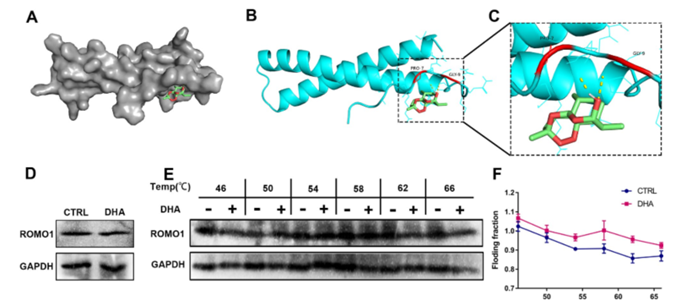
Figure 4: DHA Binding to ROMO1.
- Targeted Drug Delivery: ZIF-DHA nanoparticles exhibited efficient cellular uptake and targeted delivery, ensuring that DHA was effectively delivered to ovarian cancer cells. The encapsulation of DHA within ZIF-8 nanoparticles enhanced its solubility and stability, improving its overall therapeutic potential.
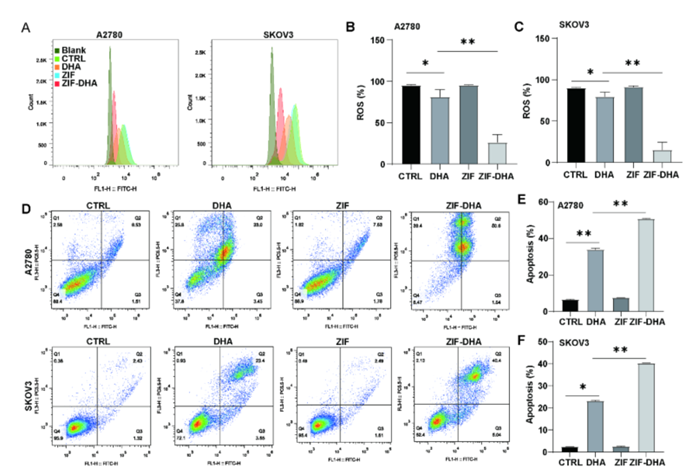
Figure 5: ZIF-DHA Suppresses ROS Production and Induces Apoptosis.
- In Vivo Validation: In vivo experiments using ovarian cancer xenograft models demonstrated that ZIF-DHA nanoparticles effectively inhibited tumor growth without causing significant toxicity to healthy tissues. This suggests that ZIF-DHA could offer a safer alternative to current chemotherapy options.
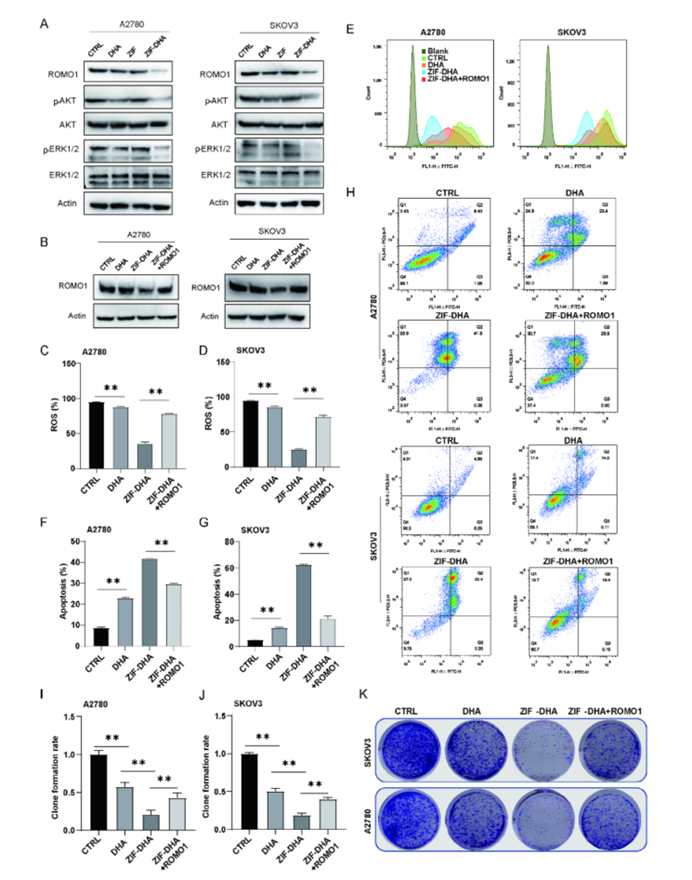
Figure 7: Cytotoxic Effects of ZIF-DHA in Ovarian Cancer Cells Dependent on ROMO1.
- ROMO1 Overexpression Reversal: The study demonstrated that overexpression of ROMO1 in ovarian cancer cells could reverse the apoptotic effects of ZIF-DHA. This finding highlights the importance of ROMO1 in the mechanism of action of ZIF-DHA and suggests that targeting ROMO1 could enhance the therapeutic efficacy of ZIF-DHA.
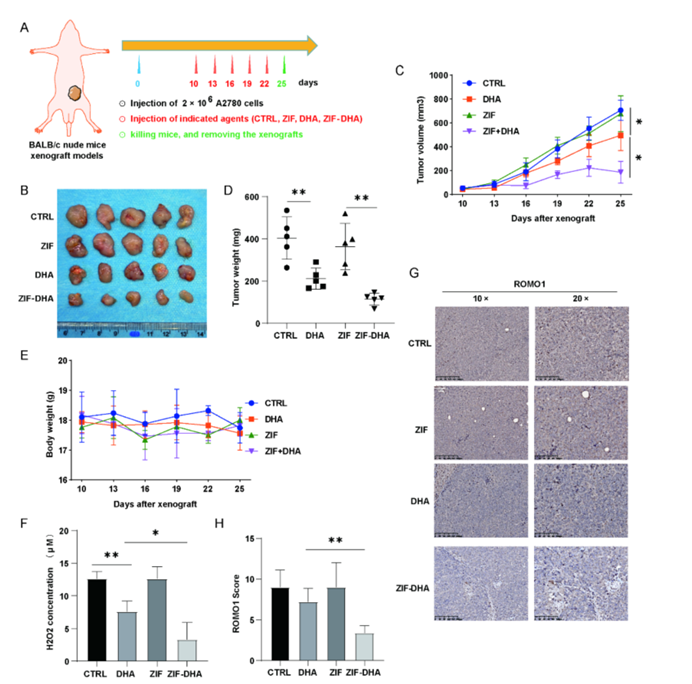
Figure 8: ZIF-DHA Inhibits ROMO1 Signaling in Ovarian Cancer Xenografts.
- Clinical Implications: The research holds significant potential for clinical applications, as it suggests that ZIF-DHA nanoparticles could provide a more effective and less toxic treatment option for ovarian cancer. The study also paves the way for the use of MOF-based drug delivery systems in treating other cancers or diseases requiring targeted therapies.
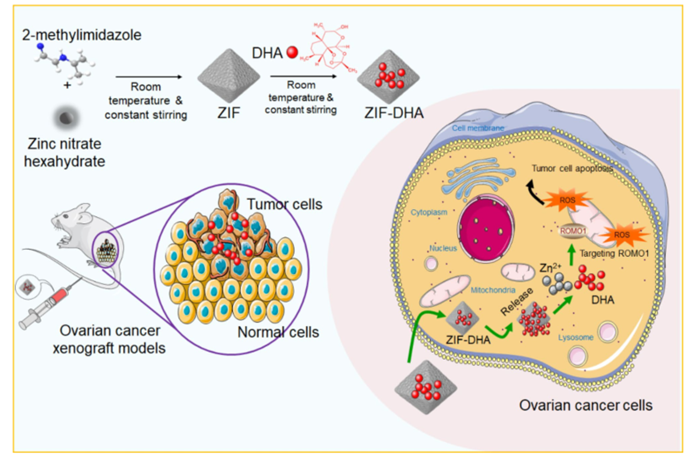
Figure 8: Schematic Representation of ZIF-DHA Nanoparticles Against Ovarian Cancer.
Implications and Future Applications
The findings of this study could have a transformative impact on the treatment of ovarian cancer. If successfully translated into clinical practice, ZIF-DHA nanoparticles may offer a more effective and less toxic alternative to current ovarian cancer therapies, improving both survival rates and quality of life for patients. Furthermore, the success of this study could lead to the development of similar nanotechnology-based drug delivery systems for other cancers, offering a broader range of applications in cancer treatment.
Continued research and development are necessary to optimize the clinical use of ZIF-DHA nanoparticles and explore their potential in combination with other therapies. The identification of ROMO1 and ROS as key therapeutic targets opens up new possibilities for precision medicine and personalized cancer therapies.
Reference
Yan, Yuanliang, et al. “Metal-Organic Framework-Encapsulated Dihydroartemisinin Nanoparticles Induces Apoptotic Cell Death in Ovarian Cancer by Blocking ROMO1-Mediated ROS Production.” Journal of Nanobiotechnology, vol. 21, no. 204, 2023, https://doi.org/10.1186/s12951-023-01959-3.
