Editor: Tiffany
Researchers have developed an optical method to temporarily increase the permeability of the blood-brain tumor barrier, enhancing the efficacy of paclitaxel treatment in glioblastoma models.
Key Highlights
- Research Question:
How can the permeability of the blood-brain tumor barrier (BBTB) be modulated to improve drug delivery for glioblastoma treatment? - Research Difficulties:
Traditional therapies face challenges with inadequate drug delivery due to the restrictive nature of the blood-brain barrier and inconsistencies between preclinical and clinical trial outcomes. - Key Findings:
The use of pulsed laser excitation of gold nanoparticles effectively and reversibly enhances BBTB permeability, allowing for significant increases in paclitaxel delivery, resulting in substantial tumor volume reduction and extended survival in animal models. - Innovative Aspects:
This method, referred to as optoBBTB, provides a non-invasive and targeted approach to drug delivery, enabling the reevaluation of previously ineffective chemotherapy agents. - Importance of the Study:
This research offers insights into potential strategies for improving glioblastoma treatment, highlighting the need for more effective therapies that can navigate the challenges presented by the blood-brain barrier.
Glioblastoma and the Blood-Brain Tumor Barrier
Glioblastoma (GBM) is classified as World Health Organization (WHO) grade IV astrocytoma and represents the most aggressive and common type of primary brain tumor. It arises from glial cells and is characterized by rapid growth, extensive infiltration into surrounding brain tissue, and a high degree of heterogeneity. Symptoms of glioblastoma can include headaches, seizures, cognitive dysfunction, and neurological deficits, significantly impacting patients’ quality of life. Current treatment options primarily involve surgical resection followed by radiation and chemotherapy, but outcomes remain poor, with median survival still around 15 months. This is largely due to the protective blood-brain barrier (BBB), which limits the delivery of effective therapeutic agents to the tumor site. Despite advances in treatment modalities, GBM continues to pose significant challenges, underscoring the urgent need for innovative strategies to improve drug delivery and therapeutic efficacy.
Research Aim & Objectives: Aiming for Better Drug Delivery
This study set out to investigate the modulation of the blood-brain tumor barrier (BBTB) to enhance therapeutic delivery for glioblastoma. Specifically, the aim was to explore the feasibility of an innovative optical technique, termed optoBBTB, for temporarily increasing BBTB permeability, thereby facilitating the effective delivery of paclitaxel, a chemotherapy agent previously abandoned for glioblastoma treatment due to its poor brain penetration. The research objectives included characterizing the intratumoral heterogeneity of BBTB in human glioblastoma, developing and validating genetically engineered mouse models that replicate key features of GBM, and assessing the efficacy of the optoBBTB method in promoting drug delivery and improving therapeutic outcomes. By bridging the gap between preclinical and clinical settings, this research aims to provide insights into potential new approaches for overcoming the challenges posed by the blood-brain barrier and ultimately improving patient prognosis in glioblastoma therapy.
The research team, consisting of Qi Cai, Xiaoqing Li, Hejian Xiong, and others from the University of Texas at Dallas and Southwestern Medical Center, published their findings in Nature Communications on July 31, 2023.
Experimental Approach: Modulating BBTB for Improved Therapeutic Efficacy
(1) Experimental Process Outline
- Characterization of human glioblastoma to analyze intratumoral BBTB heterogeneity.
- Development of genetically engineered mouse models (GEMMs) representing glioblastoma phenotypes (PS5A1 and 73 C).
- Assessment of BBTB permeability using intravenously administered EZ-link biotin and Evans blue dye.
- Administration of vascular-targeted gold nanoparticles (AuNP-BV11) followed by pulsed laser stimulation to induce optoBBTB.
- Measurement of drug delivery efficacy using paclitaxel post-optoBBTB.
- Evaluation of tumor volume reduction and survival in treated mice.
- Immunohistochemical analysis of tumor tissue for proliferation and apoptosis markers.
(2) Key Experiments
1. Assessment of BBTB Permeability in GEMMs
- Procedure: EZ-link biotin (660 Da) and Evans blue (66 kDa) were intravenously injected into the PS5A1 and 73 C GEMMs at specified days post-injection. The brains were analyzed to observe dye extravasation.
- Result: The PS5A1 model exhibited intact BBTB integrity, with no dye leakage at multiple time points, while the 73 C model showed compromised BBTB permeability at later stages.
- New Finding: The study confirmed heterogeneous BBTB permeability within glioblastoma tumors, with distinct differences in BBTB integrity between the in infiltrative and angiogenic phenotypes.
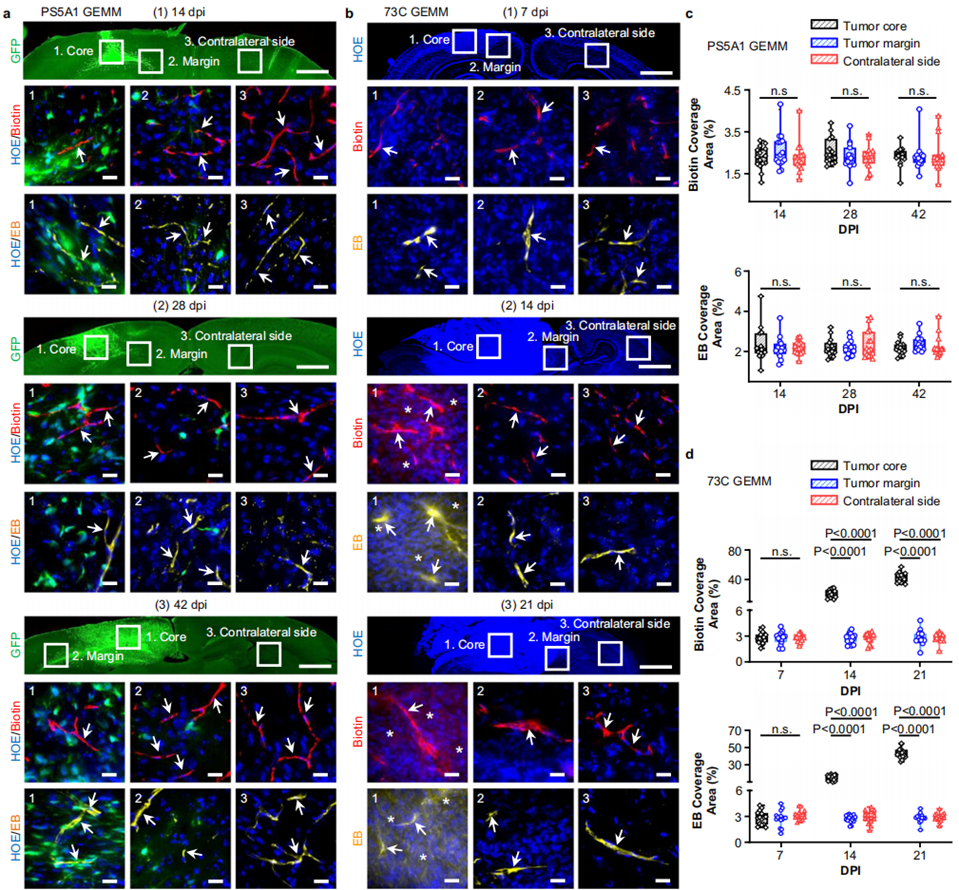
Figure 1. PS5A1 GEMM has an intact BBTB, and 73 C GEMM shows heterogeneous loss of BBTB integrity during disease progression.
2. OptoBBTB Induction and Paclitaxel Delivery
- Procedure: Following the administration of AuNP-BV11, a single pulse of a 532 nm picosecond laser was applied to the tumor region to induce optoBBTB. Paclitaxel was then administered systemically after laser stimulation.
- Result: Paclitaxel delivery increased significantly in both the tumor core and margin areas post-optoBBTB, with concentrations measured at 185 ± 92 ng/g compared to 12 ± 15 ng/g without optoBBTB.
- New Finding: The optoBBTB method effectively enhanced the brain delivery of paclitaxel, demonstrating its potential for improving therapeutic outcomes in glioblastoma treatment.
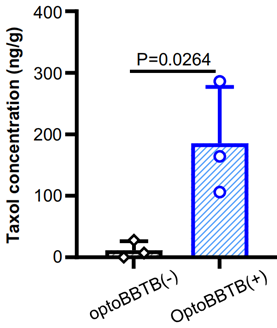
Figure 2. The analysis of Taxol concentration in the tumor without or with optoBBTB at 14 days post injection (dpi).
3. Tumor Volume Reduction and Survival Analysis
- Procedure: Mice were treated with either vehicle, paclitaxel alone, or optoBBTB followed by paclitaxel administration over three cycles. Tumor volumes were assessed at specified intervals using fluorescence imaging.
- Result: The group treated with optoBBTB and paclitaxel exhibited the smallest tumor volume (4 ± 2 mm³) and significantly prolonged median survival (60 days) compared to the control groups.
- New Finding: The combination of optoBBTB and paclitaxel resulted in marked tumor growth inhibition and enhanced survival rates, indicating the synergistic effect of the optoBBTB approach in glioblastoma therapy.
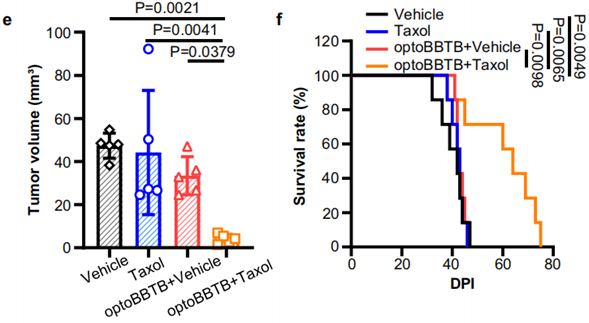
Figure 3. (e) The analysis of tumor volume by GFP fluorescent signal at 42 dpi. (f) Kaplan-Meier survival analysis.
Implications of OptoBBTB on Glioblastoma Treatment Strategies
This research presents a significant advancement in glioblastoma treatment through the application of the optoBBTB technique, which enables the precise and reversible modulation of the blood-brain tumor barrier (BBTB). This method addresses a critical challenge in glioblastoma therapy: the limited efficacy of systemic chemotherapeutic agents due to their restricted ability to penetrate the protective barrier surrounding the brain. The study demonstrates that by utilizing laser-activated vascular-targeted gold nanoparticles, optoBBTB effectively enhances the permeability of the BBTB, allowing for improved delivery of paclitaxel—an agent previously set aside due to inadequate brain penetration—into tumor tissues.
The significance of this research lies in its potential to inform treatment strategies for glioblastoma, a cancer known for its aggressive nature and poor prognosis. The findings indicate that the optoBBTB method can not only facilitate drug delivery but may also allow for the reevaluation of other potentially effective anticancer drugs that have previously been considered unsuitable for glioblastoma treatment. By bridging the gap between preclinical and clinical applications, this approach may contribute to developing more effective therapies that could enhance patient outcomes.
Additionally, the study emphasizes the value of using clinically relevant genetically engineered mouse models that accurately reflect the heterogeneity and complexity of human glioblastoma, highlighting the necessity for tailored therapeutic interventions. As further research explores the mechanisms underlying the effectiveness of optoBBTB, this technique offers a promising pathway for addressing the barriers to effective glioblastoma treatment and advancing the field of neuro-oncology.
Reference:
Cai, Qi, et al. “Optical blood-brain-tumor barrier modulation expands therapeutic options for glioblastoma treatment.” Nature communications 14.1 (2023): 4934.
