Author: Tiffany
Researchers have developed a novel oral insulin formulation utilizing silver sulfide quantum dots, which significantly reduces the risk of hypoglycemia in diabetic models. This advancement offers a new perspective in diabetes management, potentially improving the safety and convenience of insulin delivery for patients.
Key Highlights
- Research Question:
Can an innovative oral insulin formulation reduce hypoglycemic episodes while maintaining effective glycemic control? - Research Difficulties:
Previous attempts at oral insulin delivery were hampered by insulin’s instability in acidic environments and low absorption rates in the gastrointestinal tract. - Key Findings:
The new formulation, which combines insulin with silver sulfide quantum dots and a chitosan/glucose polymer, is pH-responsive, ensuring stability in acidic conditions, and it demonstrates significant glucose-lowering effects without causing hypoglycemia. - Innovative Aspects:
The oral formulation utilizes quantum dot technology for enhanced bioavailability and targeted delivery to the liver, coupled with a polymer that releases insulin in response to digestive enzymes. - Importance of the Study:
This advancement could lead to more effective and patient-friendly diabetes treatments, reducing the incidence of dangerous hypoglycemic events associated with traditional injectable insulin therapies.
Challenges in Diabetes Management
Insulin plays a pivotal role in the management of diabetes, particularly for individuals with type 1 diabetes who depend on exogenous insulin to regulate their blood glucose levels. Traditionally, insulin is administered via injections, a method that can be both inconvenient and distressing for patients, particularly children and the elderly. Moreover, the risk of hypoglycemia, a potentially life-threatening condition characterized by dangerously low blood sugar levels, remains a significant concern associated with insulin therapy. Current treatment regimens often involve a complex interplay of dietary management, exercise, and continuous glucose monitoring, which can lead to challenges in adherence. As the global prevalence of diabetes continues to rise, with an estimated 425 million individuals affected, there is an urgent need for innovative therapeutic approaches that enhance the safety and convenience of insulin delivery, thereby improving patients’ quality of life and adherence to treatment.
Research Aim & Objectives
The primary aim of this research was to develop and evaluate a novel oral insulin formulation designed to reduce the incidence of hypoglycemic episodes while effectively controlling blood glucose levels in diabetic models. The study specifically sought to address the challenges associated with traditional insulin delivery methods, such as instability in acidic environments and low absorption rates in the gastrointestinal tract. Key objectives included:
- Formulating an insulin-conjugated system using silver sulfide quantum dots and a pH-responsive chitosan/glucose polymer to enhance stability and absorption.
- Investigating the release profile of insulin from this formulation in response to digestive enzymes and varying pH levels.
- Assessing the pharmacodynamic effects of the formulation on blood glucose levels in diabetic animal models, focusing on its ability to lower glucose without inducing hypoglycemia.
- Evaluating the safety and tolerability of the oral formulation in various preclinical settings.
Through these objectives, the research aimed to provide a safer, more patient-friendly alternative to injectable insulin therapies, ultimately improving diabetes management. The team of researchers, including Nicholas J. Hunt and Glen P. Lockwood from the University of Sydney, published their findings in Nature Nanotechnology.
Experimental Design and Key Findings of the QD-INS–CS/GS Formulation
Experimental Process Outline
- Synthesis of silver sulfide quantum dots (Ag2S QDs).
- Conjugation of insulin to Ag2S QDs using EDC/NHS coupling.
- Formation of chitosan/glucose (CS/GS) copolymer for coating.
- Coating QD-INS with CS/GS via electrostatic interactions.
- Assessment of insulin release profiles using various enzymes and pH conditions.
- Evaluation of oral bioavailability and pharmacokinetics in diabetic mouse models.
- Conducting oral glucose tolerance tests (oGTT) to determine efficacy and safety.
- Testing the formulation in non-diabetic baboons for further validation.
Key Experiments Introduction
1. Release Profile of QD-INS–CS/GS
- Procedure: The release of carbon-14 (14C) insulin (14C-INS) from QD-INS–CS/GS was measured using a dual-chamber system. The chambers were separated by 10 kDa dialysis tubing to allow only free insulin to pass. Various hydrolyzing enzymes, including cellulase and β-glucosidase, were co-incubated with the formulation.
- Result: Co-incubation with β-glucosidase resulted in a 50% release of insulin within 1 hour in the second chamber, with the rate of release increasing with temperature and decreasing pH.
- New Finding: The study demonstrated that the CS/GS polymer is highly sensitive to enzymatic degradation, which effectively triggers insulin release in a controlled manner.
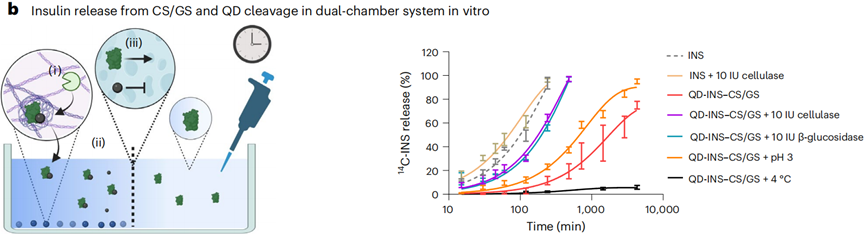
Figure 1. Release of 14C-INS from QD-14C-INS–CS/GS was examined in a dual-chamber system.
2. Pharmacodynamic Effects in Diabetic Models
- Procedure: The efficacy of oral QD-INS–CS/GS was evaluated using oral glucose tolerance tests (oGTT) in C57BL/6J mice. Mice received either subcutaneous insulin (SC-INS) or oral QD-INS–CS/GS, followed by an oral glucose administration (2 g/kg).
- Result: Both SC-INS (2 IU/kg) and oral QD-INS–CS/GS (20 IU/kg) produced similar reductions in the area under the curve (AUC) for glucose levels. High-dose (5 IU/kg) SC-INS induced hypoglycemia (BG < 3.0 mmol/l), whereas oral QD-INS–CS/GS did not cause hypoglycemia even at 300 IU/kg.
- New Finding: This experiment revealed that the oral formulation can effectively reduce blood glucose levels without inducing hypoglycemia, highlighting its potential as a safer alternative to traditional injectable insulin.
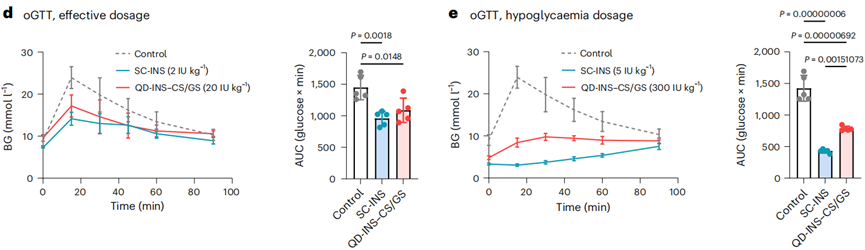
Figure 2. (d) oGTTs were performed 15 min post-administration of SC-INS or QD-INS–CS/GS. (e) Hypoglycaemia (BG < 2.9 mmol l–1) was induced by a high dosage of SC-INS (5 IU kg–1). High-dosage QD-INS–CS/GS (300 IU kg–1) did not induce hypoglycaemia.
3. Biodistribution and Targeting Studies
- Procedure: The biodistribution of subcutaneously injected insulin (SC-INS) and orally administered QD-INS–CS/GS was assessed using 14C-INS in C57BL/6J mice. Blood and various tissues (liver, muscles, fat) were collected at different time points post-administration.
- Result: At 0.5 hours, QD-INS–CS/GS showed a Cmax value of 0.06 IU/ml and a Tmax of 0.5 hours, with 20% distribution to the liver and 60% to the intestine. By 2 hours, distribution was similar to that of SC-INS.
- New Finding: The targeted delivery of the oral formulation to the liver suggests enhanced bioavailability and effectiveness compared to traditional routes, further supporting its potential in diabetes management.
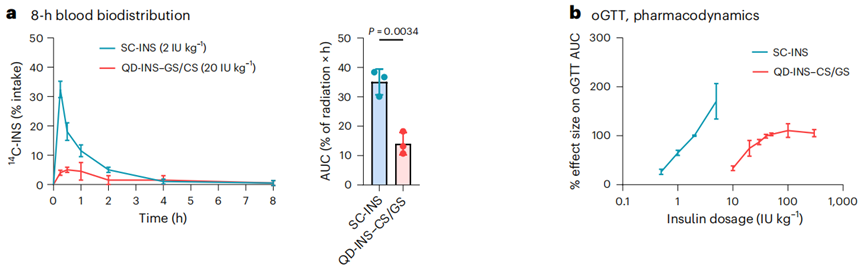
Figure 3. (a) Insulin concentration was measured at 0, 0.25, 0.50, 1.00, 2.00, 4.00 and 8.00 h following a subcutaneous (SC) injection of insulin or oral administration of QD-INS–CS/GS. (b) Pharmacodynamic effect was measured by the effect size in reducing the AUC in oGTTs.
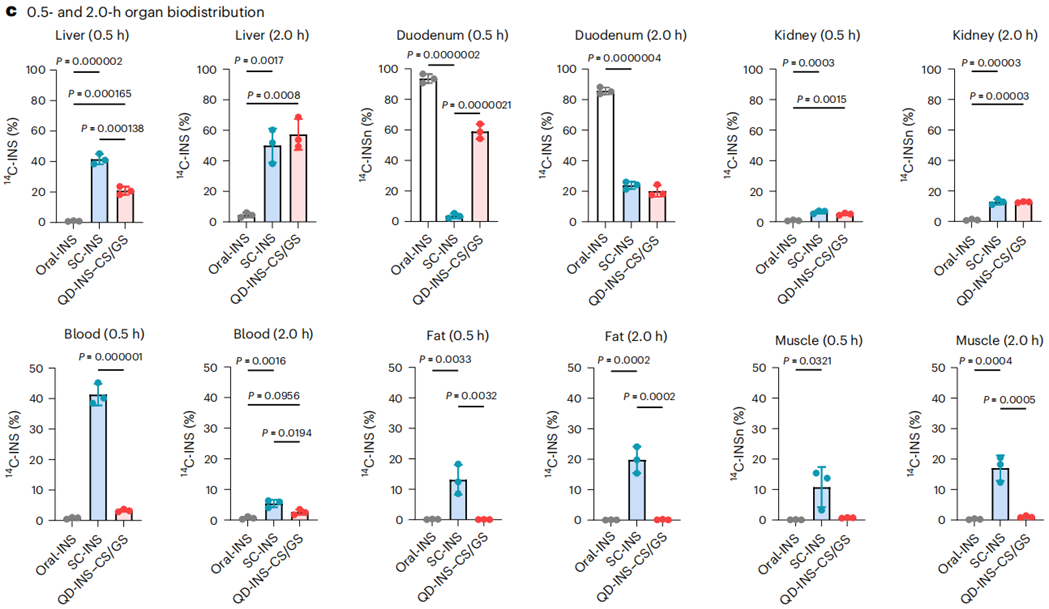
Figure 4. Biodistribution of administered 14C-INS was measured in the liver, duodenum, kidneys, blood, adipose fat and quadriceps muscle at 0.5 and 2.0 h.
Implications and Future Directions for Oral Insulin Therapy
This study presents a significant advancement in diabetes management through the development of an innovative oral insulin formulation known as QD-INS–CS/GS, which effectively combines silver sulfide quantum dots with a pH-responsive chitosan/glucose polymer. Key findings from the research demonstrate that this novel formulation can significantly lower blood glucose levels in diabetic models without causing hypoglycemic episodes, addressing a major concern associated with traditional injectable insulin therapies. The formulation’s ability to remain stable in acidic environments and release insulin in response to enzymatic activity enhances its bioavailability, particularly targeting the liver. This innovation not only offers a safer and more patient-friendly alternative for insulin delivery but also holds the potential to revolutionize the treatment of diabetes by improving adherence and overall patient quality of life.
In conclusion, the research underscores the potential of the QD-INS–CS/GS oral insulin formulation as a transformative approach in diabetes treatment. The study has successfully demonstrated that this formulation can maintain effective glycemic control while minimizing the risks of hypoglycemia—a common and dangerous side effect of traditional insulin therapies. By leveraging advanced nanotechnology, this formulation provides a promising solution to the challenges faced by patients who require insulin. The findings pave the way for future clinical trials aimed at assessing the safety and efficacy of oral insulin formulations in human populations, with the hope of improving diabetes management and patient outcomes worldwide. Additionally, the technology developed in this study could be extended to other peptide and protein therapeutics, further broadening its impact in the field of medicine.
Reference:
Hunt, Nicholas J., et al. “Oral nanotherapeutic formulation of insulin with reduced episodes of hypoglycaemia.” Nature Nanotechnology 19.4 (2024): 534-544.
