Editor: Nina
This study presents a novel near-infrared light-activatable dissolving microneedle system (MN-pB/I) that integrates reactive oxygen species-responsive chemotherapy and phototherapy for targeted and effective melanoma treatment, demonstrating significant tumor inhibition with minimal side effects.
Key Preview
Research Question
The primary research question addressed in this study is: How can the efficacy of transdermal drug delivery systems be improved for melanoma treatment through controlled drug release and effective imaging techniques?
Research Design and Strategy
The researchers designed a microneedle system capable of delivering chemotherapy and phototherapy in a controlled manner. They focused on overcoming challenges faced by existing transdermal drug delivery methods, such as inadequate drug penetration and activation. The MN-pB/I system was constructed using dissolvable polyvinylpyrrolidone (PVP) microneedles containing liposomes co-loaded with indocyanine green (ICG) and a ROS-responsive prodrug of doxorubicin (pB-DOX).
Method
The method involved the synthesis of the pB-DOX prodrug and the preparation of liposomes encapsulating both the prodrug and ICG. The MN system was applied to melanoma tumor sites in preclinical models, followed by NIR light exposure to activate the drug release and treatment processes. Multiple assays and imaging techniques were employed to evaluate the drug delivery efficiency and anti-tumor effects.
Key Results
The MN-pB/I system resulted in a 93.5% inhibition of tumor growth, significantly outperforming control groups. The localized drug delivery minimized systemic side effects, and the use of imaging techniques allowed for effective monitoring of treatment progress. The study reported negligible treatment-induced side effects and cardiotoxicity.
Significance of the Research
This research is significant as it presents a multimodal, biocompatible theragnostic system that integrates chemotherapy, photodynamic therapy, and imaging. The MN-pB/I system represents a step forward in melanoma treatment, addressing the limitations of traditional methods by providing targeted, controlled, and effective therapies.
Introduction
Melanoma is a highly malignant form of skin cancer that poses significant treatment challenges due to its aggressive nature and potential for metastasis. While conventional therapies such as surgery and chemotherapy exist, they often come with severe systemic side effects and limitations when treating lesions in sensitive areas. As a result, there is a pressing need for innovative treatment strategies that can effectively target melanoma while minimizing adverse effects.
Recent developments in nanocarrier-based transdermal drug delivery systems (NTDDSs) offer a promising alternative, allowing for localized treatment that bypasses systemic circulation. However, traditional NTDDSs have struggled with issues related to drug release control and limited penetration depth into the skin. This study aims to address these challenges by introducing a near-infrared (NIR) light-activatable dissolving microneedle system (MN-pB/I) that combines ROS-responsive chemotherapy with phototherapy. The central research question focuses on how to enhance the efficacy of drug delivery for melanoma treatment through this innovative approach.
Research Team and Objective
The research team comprised Fan Liu, Zeneng Cheng, and Hanxi Yi from Central South University. Their paper, titled “NIR light-activatable dissolving microneedle system for melanoma ablation enabled by a combination of ROS-responsive chemotherapy and phototherapy,” was published in the Journal of Nanobiotechnology. The objective of this research was to develop a microneedle system that allows for controlled drug release and effective imaging guidance, ultimately improving treatment outcomes for melanoma patients.
Experimental Process
Experiment 1: Fabrication of MN-pB/I
Key Steps:
- Preparation of the Prodrug: The ROS-responsive doxorubicin prodrug (pB-DOX) was synthesized by incorporating a boronate moiety into the structure of doxorubicin. This modification reduces the toxicity of doxorubicin to normal cells until activated by reactive oxygen species (ROS).
- Liposome Formation: Liposomes were created by co-loading pB-DOX and the photosensitizer indocyanine green (ICG). The liposomes were prepared using a thin-film hydration method followed by sonication to ensure uniform size and encapsulation.
- Microneedle Array Preparation: An aqueous polyvinylpyrrolidone (PVP) solution (40%) was prepared and the liposomal mixture was introduced into PDMS molds to form MNs. The solvent was evaporated under vacuum to create dissolvable MNs containing the loaded liposomes.
- Patch Peeling and Storage: After drying, the MN patches were peeled off from the molds and stored in a desiccator to maintain stability until further use.
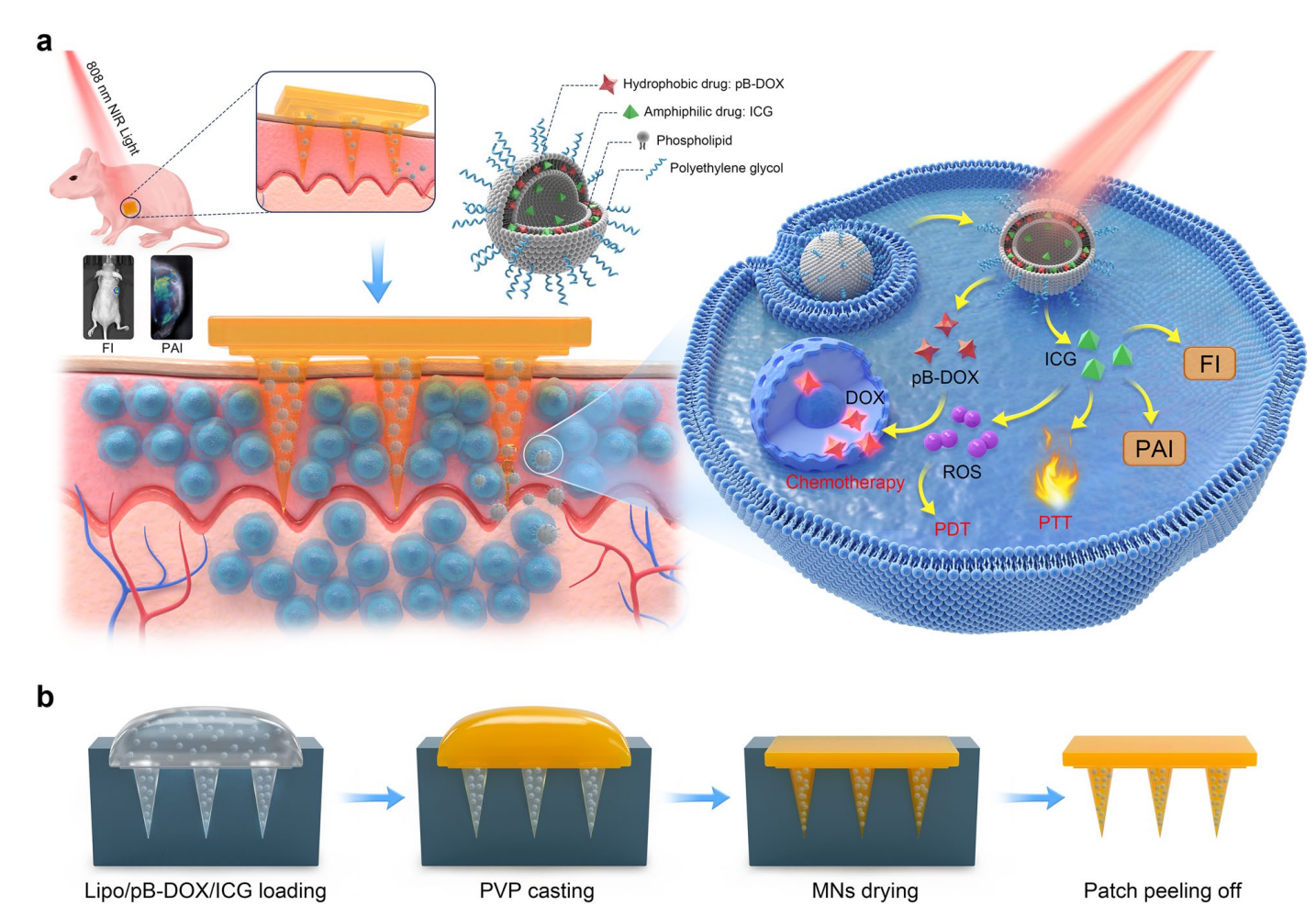
Scheme 1. Design principle of the NIR light-activatable dissolving MN system (MN-pB/I) for multimodal theragnostic application in melanoma.
Results and Key Data:
- The MN-pB/I patches exhibited uniform size and morphology with a 10 × 10 needle array arrangement.
- Quantitative analysis revealed that each MN patch contained approximately 5.03 μg of pB-DOX.
Significance of the Result:
This experiment established a novel microneedle system capable of delivering dual therapeutic agents (pB-DOX and ICG) directly to melanoma tissues, overcoming the limitations of conventional drug delivery systems that often suffer from poor skin penetration and low bioavailability.
Key Innovations and Advantages:
- Integration of Dual Therapies: The MN-pB/I system uniquely combines chemotherapy (via pB-DOX) with photodynamic therapy (via ICG), enhancing therapeutic efficacy.
- Controlled Release Mechanism: The ROS-responsive nature of pB-DOX allows for targeted drug activation at the tumor site, minimizing systemic exposure and potential side effects associated with traditional delivery mechanisms.
Experiment 2: Drug Release and Imaging
Key Steps:
- Application of MN-pB/I Patches: The MN-pB/I patches were applied to the tumor sites in A375 melanoma-bearing mice, allowing the microneedles to penetrate the skin.
- NIR Light Activation: Following the application, the tumor sites were irradiated with near-infrared (NIR) light to activate ICG, which subsequently generated ROS.
- Monitoring Drug Release: The release of pB-DOX from the liposomes was monitored through fluorescence and photoacoustic imaging, enabling real-time tracking of drug distribution within the tumor.
Results and Key Data:
- The imaging results demonstrated that the liposomes released their payload efficiently into the tumor interstitial fluid upon application and activation.
- Fluorescence imaging indicated a significant accumulation of the drugs within the tumor, confirming effective penetration and distribution.
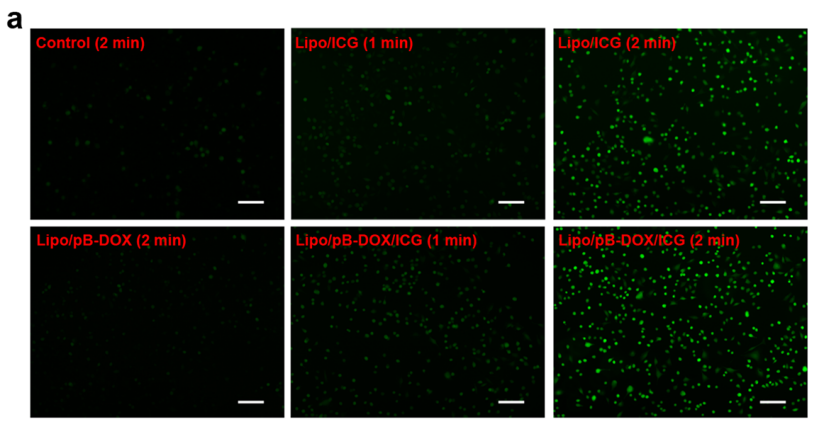
Figure 1. Fluorescence images of A375 cells containing Lipo/pB-DOX, Lipo/ICG, and Lipo/pB-DOX/ICG after intracellular ROS generation by laser irradiation (1.0 W/cm2 ) for 1 or 2 min (scale bar: 200 μm).
Significance of the Result:
This step validated the MN-pB/I system’s capability for localized drug release and real-time monitoring of treatment, which is critical for assessing therapeutic outcomes in real-time and adjusting treatment protocols accordingly.
Key Innovations and Advantages:
- Real-Time Imaging: The combination of fluorescence and photoacoustic imaging techniques provides a powerful tool for visualizing drug distribution, which is often lacking in traditional transdermal drug delivery systems.
- Enhanced Drug Activation: The NIR light-activated release mechanism allows for precise control over when and where the drug is activated, improving therapeutic efficacy while reducing side effects.
Experiment 3: In Vivo Efficacy Testing
Key Steps:
- Establishing Tumor Models: A375 melanoma cells were subcutaneously injected into BALB/c nude mice to establish the tumor model.
- Treatment Administration: Once the tumors reached approximately 150-200 mm³ in volume, the MN-pB/I patches were applied, followed by NIR laser irradiation.
- Monitoring Tumor Growth: Tumor volumes and body weights were recorded over a 15-day period to evaluate the therapeutic effectiveness of the MN-pB/I treatment.
Results and Key Data:
- The MN-pB/I + laser treatment group achieved a remarkable tumor growth inhibition rate (IRT) of 93.5%, significantly outperforming control groups.
- Visual observations and tumor weight measurements indicated complete tumor ablation in some cases after a single dose.
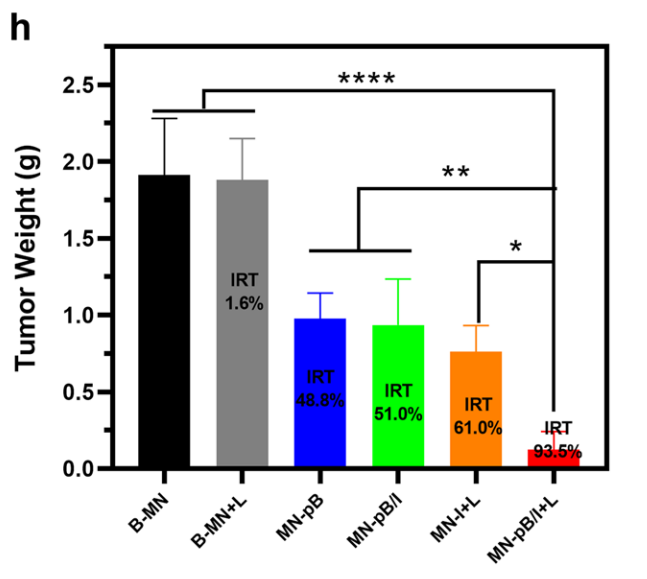
Figure 5. Tumor weights of each group at the end of the experiment and IRT.
Significance of the Result:
The results underscore the potential of the MN-pB/I system to deliver effective treatments against melanoma while maintaining a favorable safety profile, with negligible systemic side effects or cardiotoxicity.
Key Innovations and Advantages:
- Single-Dose Efficacy: The ability to achieve significant tumor inhibition with a single administration of MN-pB/I is a notable advancement over traditional therapies that often require multiple cycles and result in cumulative toxicities.
- Multimodal Treatment Approach: The integration of chemotherapy, photodynamic therapy, and imaging in a single platform presents a novel strategy for treating melanoma, representing a substantial improvement over existing mono-modal treatments that lack such versatility.
Conclusion
The study successfully demonstrated that the MN-pB/I system provides a novel approach for melanoma treatment, achieving significant tumor ablation through a single dose. The findings highlight the potential of this integrated system to combine chemotherapy and phototherapy, allowing for targeted treatment with minimal side effects. However, the research acknowledges limitations, including the need for further clinical studies to establish long-term efficacy and safety. Future research could explore the application of this technology to other types of cancers and optimize the drug delivery system for broader therapeutic use.
Reference:
Liu, Fan, Zeneng Cheng, and Hanxi Yi. “NIR light-activatable dissolving microneedle system for melanoma ablation enabled by a combination of ROS-responsive chemotherapy and phototherapy.” Journal of Nanobiotechnology 21.1 (2023): 61.
