Editor: Sarah
A recent study by Yonsei University and the Korea Institute of Radiological and Medical Sciences presents an innovative approach to treating triple-negative breast cancer (TNBC), a particularly aggressive form of cancer. This research focuses on erythrocyte membrane nanoparticles (EDNs), which utilize red blood cell membranes to directly deliver therapeutic agents to cancer cells. The study demonstrates how this approach not only enhances treatment effectiveness but also allows for real-time diagnostic imaging.
By encapsulating doxorubicin (DOX), a chemotherapy drug, within these erythrocyte-derived nanoparticles, researchers have developed a dual-action therapy capable of both targeting and monitoring tumor growth. Anti-EGFR antibodies are incorporated on the surface of EDNs, allowing for precise drug delivery specifically targeting EGFR-positive TNBC cells. This strategy enhances treatment efficacy while minimizing side effects.
Contributions and Key Findings
- Development of EDNs for TNBC Treatment:
This study introduces erythrocyte-derived nanoparticles (EDNs) as an innovative delivery system for anti-cancer therapies, offering a new way to treat TNBC. By leveraging the flexible and simple structure of red blood cell membranes, EDNs are shown to have significant advantages in systemic circulation, remaining in the bloodstream for extended periods.
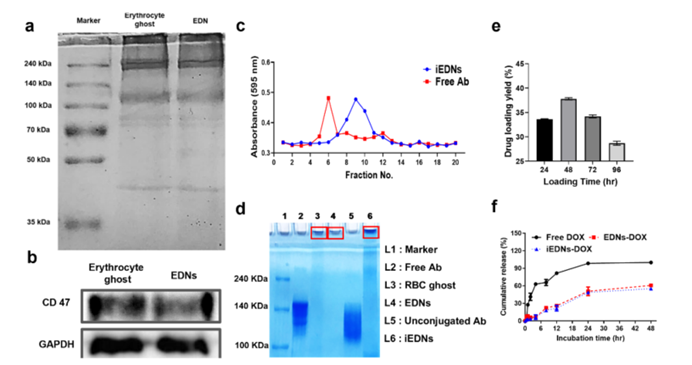
Figure 1: Preparation and characterization of EDNs caption.
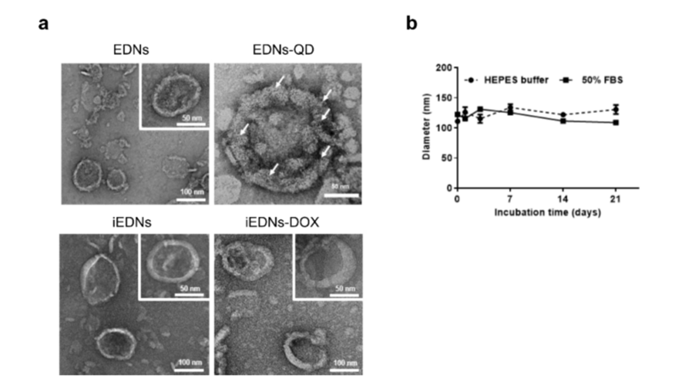
Figure 2: Changes in the morphologies and sizes of EDNs.
- Encapsulation of Doxorubicin (DOX):
DOX, a commonly used chemotherapy drug, was successfully encapsulated within EDNs. This method of encapsulation minimizes the toxic side effects typically associated with DOX while enhancing its targeted delivery to cancer cells.
- Targeted Drug Delivery Using Anti-EGFR Antibodies:
The addition of anti-EGFR antibodies on the surface of EDNs allows the nanoparticles to specifically target EGFR-positive TNBC cells. In preclinical tests, this modification resulted in significantly improved tumor targeting and reduced liver uptake compared to untargeted nanoparticles. The use of anti-EGFR antibodies helps guide the EDNs directly to tumor sites, enhancing the precision of treatment.
- Improved Tumor Growth Inhibition:
In animal studies, mice treated with anti-EGFR-targeted EDNs encapsulating DOX showed significantly reduced tumor growth compared to those treated with conventional chemotherapy. This result highlights the potential of EDNs to offer more effective treatment options for aggressive cancers like TNBC.
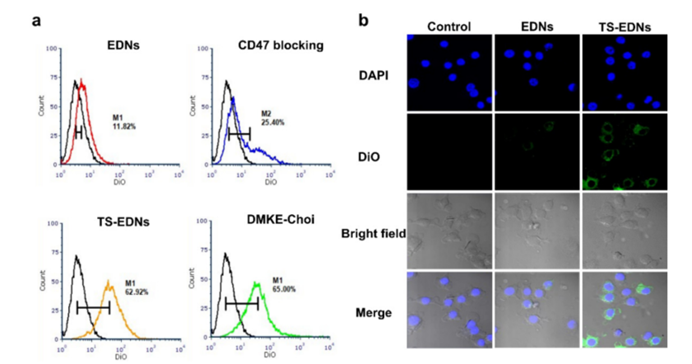
Figure 3: Interaction of EDNs with macrophages caption
- Combination of Drug Delivery and Diagnostic Imaging:
Researchers incorporated quantum dots (QDs) into the EDNs, enabling the nanoparticles to serve as both therapeutic agents and diagnostic tools. This dual functionality allows for real-time tumor imaging, which could be used to monitor the progression of treatment and detect tumors at earlier stages. The ability to visualize tumors in real-time through QD fluorescence imaging represents a major advancement in cancer diagnostics.
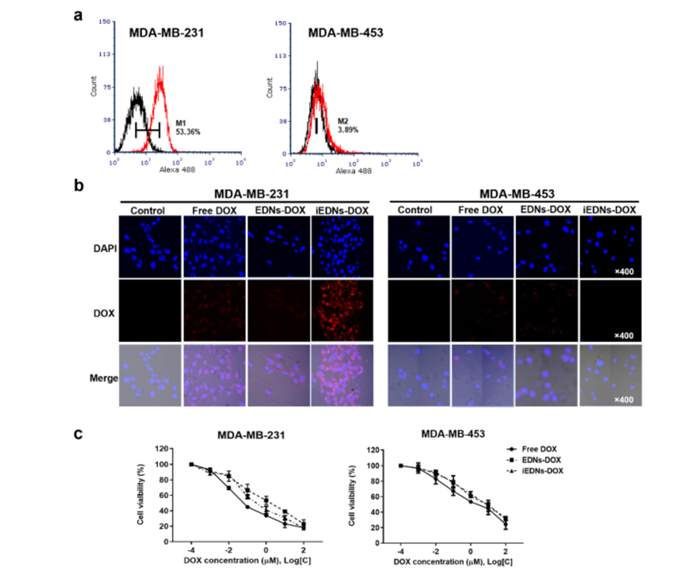
Figure 4: Tumor‐targeted intracellular drug delivery and anti‐cancer effects of iEDNs‐DOX.
- High Biocompatibility and Immune Evasion:
EDNs exhibit high biocompatibility, thanks in part to the presence of CD47, a “don’t eat me” signal that prevents detection by the immune system. This feature ensures that the nanoparticles remain in circulation long enough to target tumors effectively. The immune evasion properties of EDNs reduce the likelihood of premature clearance by the body’s defense systems.
- Efficient Delivery and Tumor Localization:
In vitro and in vivo experiments demonstrated that EDNs, particularly those targeted with anti-EGFR antibodies, efficiently deliver drugs to tumor cells while minimizing off-target effects. The encapsulation of DOX in EDNs also showed a slower and more controlled release of the drug, which could potentially reduce the drug’s toxic effects on healthy tissues.
- Promising Preclinical Results for Clinical Translation:
The study’s findings underscore the potential of EDNs as a powerful tool for the treatment of TNBC. The ability to both treat and image tumors makes EDNs a promising candidate for clinical applications in personalized cancer therapy. As the research progresses towards clinical trials, EDNs could provide a more effective and less invasive treatment option for patients with TNBC.
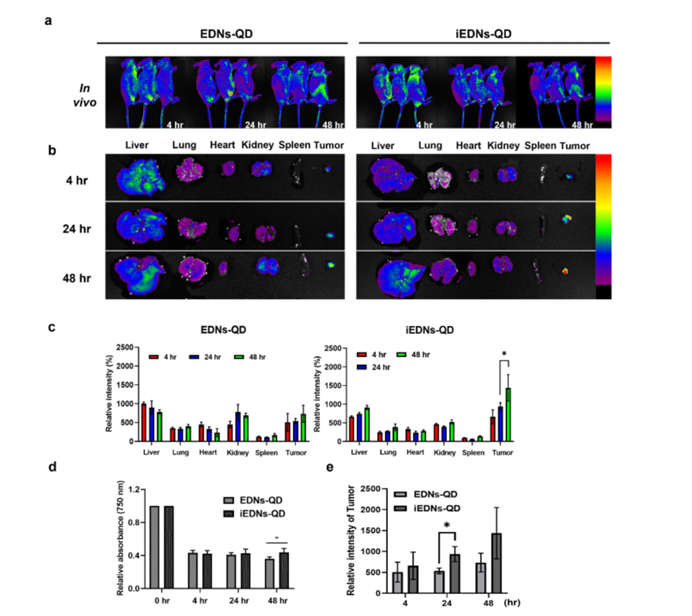
Figure 5: In vivo and ex vivo analyses of the biodistribution of iEDNs‐QDs.
Conclusion
This study demonstrates the significant potential of erythrocyte membrane nanoparticles as a dual-purpose platform for both cancer therapy and diagnostics. By combining targeted drug delivery with real-time imaging capabilities, this approach could offer a more precise and less toxic alternative to traditional chemotherapy. As further research progresses and clinical trials are conducted, EDNs may emerge as an effective and less invasive treatment option for patients with triple-negative breast cancer, paving the way for more personalized and targeted therapies.
Reference
Choi, Moon Jung, et al. “Tumor-Targeted Erythrocyte Membrane Nanoparticles for Theranostics of Triple-Negative Breast Cancer.” Pharmaceutics, vol. 15, no. 2, 2023, p. 350. MDPI, https://doi.org/10.3390/pharmaceutics15020350.
