Author: Tiffany
Researchers have developed a near-infrared nanotheranostic system that effectively targets both toxic amyloid-beta plaques and harmful inflammation in Alzheimer’s disease, offering new hope for treatment.
Key Highlights:
- Research Question:
Can a dual-targeting therapy effectively address both amyloid-beta plaques and inflammation in Alzheimer’s disease? - Research Difficulties:
Achieving effective penetration through the blood-brain barrier (BBB) and ensuring specificity in therapeutic action have posed significant challenges. - Key Findings:
The new nanotheranostic system demonstrated the ability to inhibit amyloid-beta fibril formation, degrade existing plaques, and alleviate inflammation, resulting in improved cognitive function in mice models. - Innovative Aspects:
The study introduces a near-infrared-II aggregation-induced emission (AIE) nanotheranostic capable of real-time monitoring and treatment of Alzheimer’s disease. - Importance of the Study:
This research addresses critical limitations of current Alzheimer’s treatments and paves the way for more effective therapies against neurodegenerative diseases.
Understanding Alzheimer’s Disease Pathology and Treatment Challenges
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that primarily affects older adults, leading to significant cognitive decline, memory loss, and impaired daily functioning. The disease is characterized by the accumulation of toxic amyloid-beta (Aβ) plaques and the presence of neuroinflammation, both of which contribute to neuronal damage and synaptic loss. Current therapeutic options, such as acetylcholinesterase inhibitors and monoclonal antibodies, often provide limited efficacy and specificity, failing to effectively target the dual pathology of AD. Furthermore, these treatments struggle with poor blood-brain barrier (BBB) penetration, increasing the urgency for innovative approaches that can target both Aβ plaques and inflammatory processes. The development of a dual-targeting nanotheranostic system could revolutionize AD treatment, offering a more effective and safer therapeutic strategy for managing this complex disease.
Development of a Dual-Targeting NIR-II Nanotheranostic System
The primary aim of this research was to develop a novel near-infrared (NIR)-II nanotheranostic system capable of simultaneously targeting the dual hallmarks of Alzheimer’s disease: toxic amyloid-beta (Aβ) plaques and associated neuroinflammation. The study’s objectives included synthesizing two therapeutic aggregation-induced emission (AIE) molecules and co-assembling them into a nanocomposite that enhances blood-brain barrier (BBB) penetration while maintaining high specificity for Aβ plaques. Furthermore, the research sought to investigate the controlled release of therapeutic agents triggered by reactive oxygen species (ROS) in the AD microenvironment, assess the efficacy of the nanotheranostic system in inhibiting Aβ fibril formation and degradation, and evaluate its overall therapeutic effect on cognitive function in an established animal model of Alzheimer’s disease. The research team, led by Jiefei Wang and colleagues from various institutions, published their findings in Nature Communications on January 2, 2024. By addressing these objectives, the study aimed to establish a foundation for more effective and targeted therapies for AD, ultimately improving patient outcomes and quality of life.
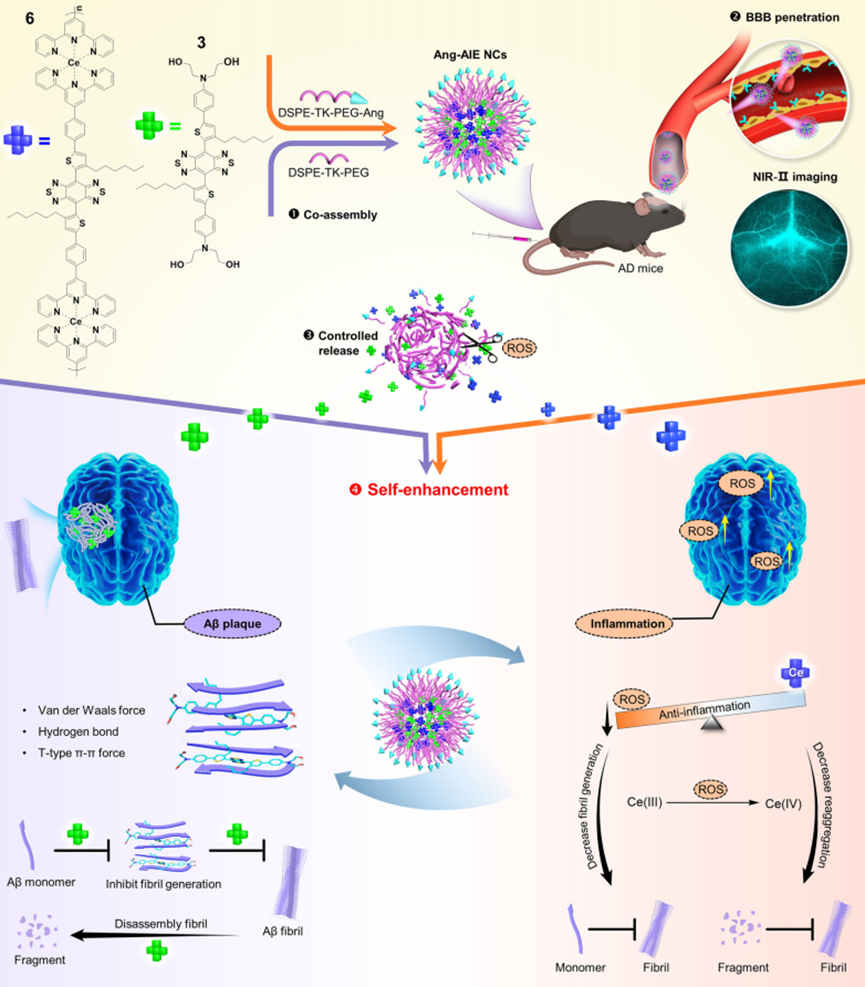
Figure 1. Schematic of NIR-II brain-target theranostic system for dual-target therapy of AD via a four-step route.
Experimental Validation of Ang-NCs: Mechanisms and Efficacy
Experimental Process Outline
- Synthesis of two therapeutic aggregation-induced emission (AIE) molecules (compound 3 and compound 6).
- Co-assembly of AIE molecules into a near-infrared (NIR)-II nanotheranostic system (Ang-NCs).
- Functionalization of the nanocomposite with angiopep-2 (Ang-2) for enhanced blood-brain barrier (BBB) penetration.
- In vitro assessments of cytotoxicity and BBB permeability using various cell lines (PC12, HT-22, etc.).
- Evaluation of the controlled release of therapeutic agents in response to reactive oxygen species (ROS).
- In vivo imaging studies using NIR-II fluorescence to monitor BBB penetration and binding to Aβ plaques in mice.
- Behavioral and cognitive assessments in APP/PS1 transgenic Alzheimer’s disease mice after treatment with Ang-NCs.
- Histological analysis of brain tissue to evaluate Aβ plaque degradation and inflammation levels.
Key Experiments
1. Controlled Release Experiment
- Procedure: The Ang-NCs were incubated with hydrogen peroxide (H2O2) to simulate the high-inflammation environment typical of Alzheimer’s disease. The size and morphology changes of the Ang-NCs were monitored using dynamic light scattering (DLS) and transmission electron microscopy (TEM).
- Result: The size of Ang-NCs increased over time when treated with H2O2, indicating effective ROS-responsive release, as evidenced by swelling and morphological changes. The DLS analysis showed a gradual increase in size, confirming the release mechanism.
- New Finding: This experiment demonstrated that the nanotheranostic system could effectively respond to the oxidative environment present in Alzheimer’s disease, facilitating the controlled release of therapeutic agents.
2. In Vivo NIR-II Imaging Experiment
- Procedure: APP/PS1 mice were intravenously injected with Ang-NCs, and NIR-II fluorescence imaging was performed at 1350 nm to track the distribution and binding of the nanocomposite in the brain over time.
- Result: The imaging revealed that Ang-NCs exhibited significant fluorescence intensity in the brain, indicating successful BBB penetration and binding to Aβ plaques. The fluorescence intensity at 1350 nm was notably higher in the Ang-NCs group compared to the control group (NCs).
- New Finding: This experiment established that the Ang-NCs could effectively cross the BBB and target Aβ plaques in vivo, which is critical for the therapeutic efficacy of the proposed nanotheranostic system.
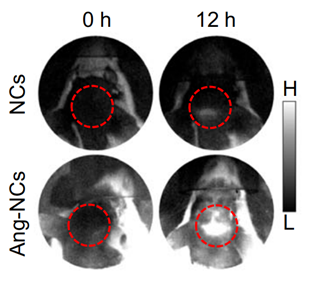
Figure 2. NIR imaging at 1350 nm of the brain in APP/PS1 mice at various time points (0 and 12 h) post i.v. injection of NCs and Ang-NCs (dosage: 10 mg/kg).
3. Cognitive Assessment Experiment
- Procedure: Cognitive function in APP/PS1 mice was evaluated using the Morris water maze (MWM) test after six intravenous injections of Ang-NCs. The escape latency, crossing number, and time spent in the target quadrant were recorded.
- Result: The Ang-NCs group showed significantly improved performance compared to the AD control group, with a notable decrease in escape latency (P = 0.0001) and increased crossing number (P < 0.0001). The residence time in the target quadrant was also significantly higher in the Ang-NCs group compared to AD mice.
- New Finding: This experiment indicated that treatment with Ang-NCs significantly enhances cognitive function and memory retention in an Alzheimer’s disease model, suggesting the potential of this dual-targeting therapy for therapeutic intervention.
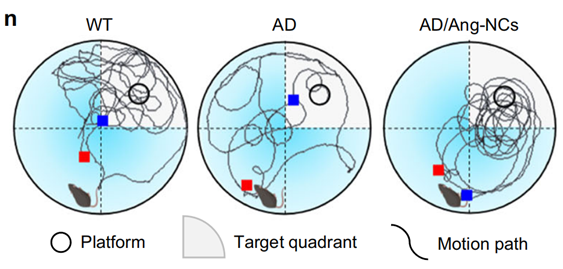
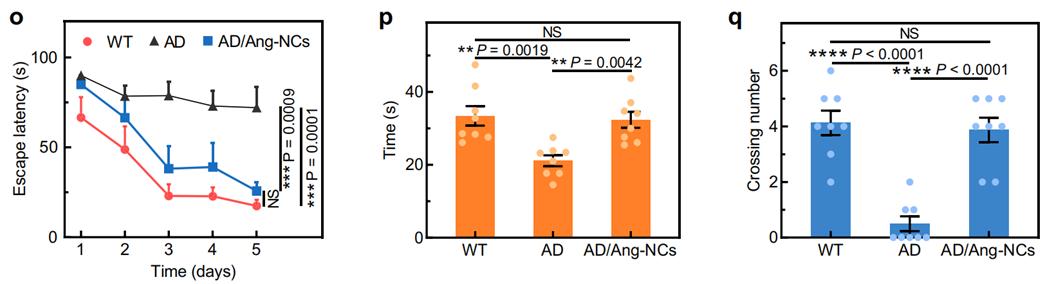
Figure 3. (n) Swimming tracks of various groups. (o–q) were respectively the escape latency.
Implications for Future Alzheimer’s Disease Therapies
The researchers emphasize the complexity of Alzheimer’s disease, marked by the interplay between amyloid-beta (Aβ) plaque accumulation and neuroinflammation. They highlight the limitations of current therapies, which often inadequately target both aspects and face challenges with blood-brain barrier (BBB) penetration. In contrast, their innovative near-infrared (NIR)-II nanotheranostic system, Ang-NCs, effectively enhances BBB permeability while specifically targeting Aβ plaques and inflammation.
Ang-NCs facilitate real-time imaging of therapeutic action and enable controlled release of agents in response to reactive oxygen species (ROS) within the Alzheimer’s microenvironment. This dual-targeting strategy significantly improves cognitive and behavioral outcomes in animal models, representing a promising shift in neurodegenerative disease treatment.
The researchers advocate for developing nanotheranostics that incorporate specificity, multi-targeting capabilities, and responsiveness to disease features. Such advancements could lead to more effective and safer therapies, ultimately enhancing patient quality of life and offering new hope against Alzheimer’s disease and other neurodegenerative disorders
Reference:
Wang, Jiefei, et al. “A one-two punch targeting reactive oxygen species and fibril for rescuing Alzheimer’s disease.” Nature Communications 15.1 (2024): 705.
