Editor: Nina
This study demonstrates the enhanced efficacy of urease-powered nanobots in delivering radionuclide therapy for bladder cancer, achieving significant tumor accumulation and nearly 90% reduction in tumor size, thereby presenting a promising advancement in targeted cancer treatment.
Key Preview
Research Question
The study investigates whether urease-powered nanobots can enhance the therapeutic efficacy of radionuclide therapy for bladder cancer by improving the accumulation and penetration of treatment agents at tumor sites.
Research Design and Strategy
The research employs an orthotopic mouse model of bladder cancer to evaluate the effectiveness of radiolabeled mesoporous silica-based urease-powered nanobots. The study combines in vivo and ex vivo imaging techniques, including positron emission tomography (PET) and polarization-dependent scattered light-sheet microscopy, to assess nanobot behavior and therapy outcomes.
Method
The researchers synthesized urease-powered nanobots and administered them intravesically in a bladder cancer model. Accumulation at the tumor site was quantified using PET imaging, while therapeutic effects were evaluated based on tumor size reduction after radionuclide therapy.
Key Results
The study found an eightfold increase in nanobot accumulation at the tumor site, with approximately 90% reduction in tumor size using intravesically administered radio-iodinated nanobots, highlighting the efficacy of this innovative delivery system.
Significance of the Research
This research represents a significant advancement in bladder cancer treatment by utilizing nanobot technology, which could enhance drug delivery and efficacy while minimizing side effects, potentially transforming therapeutic approaches for this challenging cancer type.
Introduction
Bladder cancer remains a major health concern, with a high recurrence rate following conventional treatments like intravesical immunotherapy and chemotherapy. Traditional methods often face issues such as low therapeutic efficacy due to poor drug diffusion and retention within the bladder. Recent advancements in nanotechnology have led to the development of self-propelled nanoparticles, or nanobots, that can navigate the complex biological environment of the bladder. The current study introduces a novel approach utilizing urease-powered nanobots to enhance the delivery of radionuclide therapy directly to bladder tumors. This innovation addresses the pressing need for more effective treatment options in bladder cancer management.
Research Team and Objective
The research team consists of prominent scholars and scientists, including Cristina Simó, Meritxell Serra-Casablancas, and Samuel Sánchez, among others, from various institutions, including CIC biomaGUNE and the Institute for Bioengineering of Catalonia. Conducted between March 2023 and November 2023, the study titled “Urease-powered nanobots for radionuclide bladder cancer therapy” was published in Nature Nanotechnology. The primary objective of the research was to explore the potential of urease-powered nanobots as effective carriers for radionuclide therapy in bladder cancer, aiming for higher accumulation and deeper penetration into tumor tissues.
Experimental Process
1. Nanobot Fabrication
Key Steps:
- Mesoporous silica nanoparticles (MSNPs) were synthesized using a modified Stöber method, with the addition of triethanolamine, water, and hexadecyltrimethylammonium bromide (CTAB) heated to 95°C. Tetraethyl orthosilicate was then added dropwise, and the mixture reacted for 2 hours.
- The MSNPs were functionalized with amine groups by treating them with aminopropyltriethoxysilane (APTES) and subsequently activated with glutaraldehyde to allow conjugation with urease and heterobifunctional polyethylene glycol (PEG).
- Gold nanoparticles (AuNPs) were attached to the nanobots’ surfaces to facilitate imaging.
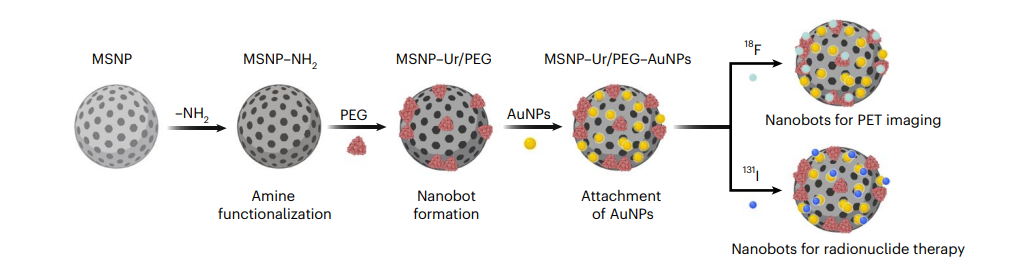
Figure 1. Schematic representation of the nanobot fabrication process and radiolabelling. Ur, urease.
Result and Key Data:
The resulting urease-powered nanobots exhibited a hydrodynamic radius of approximately 450 nm, with a zeta potential of -34.5 mV, indicating good stability in physiological conditions.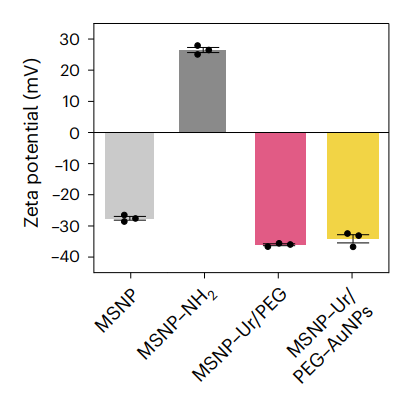
Figure 2. zeta potential (n = 3, technical replicates) Data are presented as mean values and error bars represent the s.e.m.
Significance of the Result:
This step established a robust and biocompatible delivery system capable of propulsion in the bladder environment, setting the foundation for effective drug delivery.
Key Innovations:
The integration of urease with MSNPs and AuNPs resulted in a novel nanobot design that can navigate complex biological fluids, a significant advancement over traditional drug delivery methods.
2. In Vitro Dynamics
Key Steps:
- The motion dynamics of the nanobots were evaluated in vitro by placing them in solutions containing varying concentrations of urea (0 mM and 300 mM).
- High-speed optical microscopy captured their movement, and pixel intensity distributions were analyzed to quantify their collective behavior.
Result and Key Data:
In the presence of 300 mM urea, the nanobots exhibited vigorous movement, forming active fronts and 3D vortices, while in urea-free solutions, they sedimented and displayed passive diffusion patterns. Quantitative analysis showed a significant increase in displacement of tracer particles alongside the moving nanobots.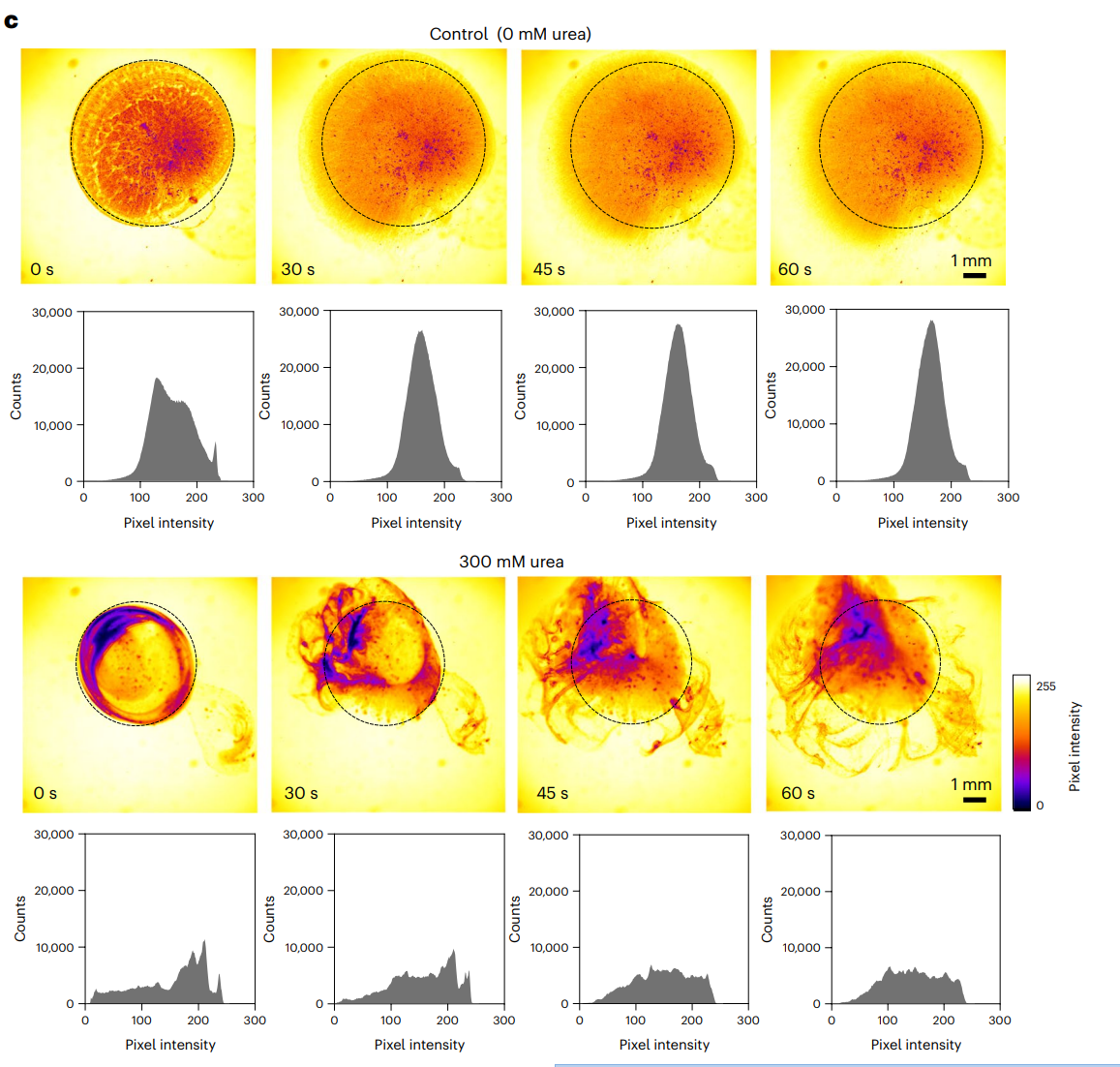
Figure 3. Snapshots depicting the nanobot motion dynamics in the absence and presence (300 mM) of urea as fuel, and corresponding pixel intensity histograms for the ROI marked by a circle.
Significance of the Result:
These findings demonstrated that urease-powered nanobots could effectively enhance diffusion and mixing within the bladder, overcoming limitations of conventional therapeutic agents that often sediment.
Key Innovations:
The study showcased the first instance of chemically powered nanobots exhibiting coordinated motion in biological fluids, paving the way for their application in targeted therapy.
3. Establishment of the Orthotopic Murine Model of Bladder Cancer
Key Steps:
- C57BL/6JRj female mice were anesthetized, and a bladder cancer model was established by intravesical instillation of MB49 bladder cancer cells.
- Tumor growth was monitored through MRI on days 7 and 14 post-implantation.
Result and Key Data:
MRI imaging confirmed the establishment of tumors, with distinct hypointense areas indicating tumor presence. Tumor volumes were quantified and used for subsequent treatment group randomization.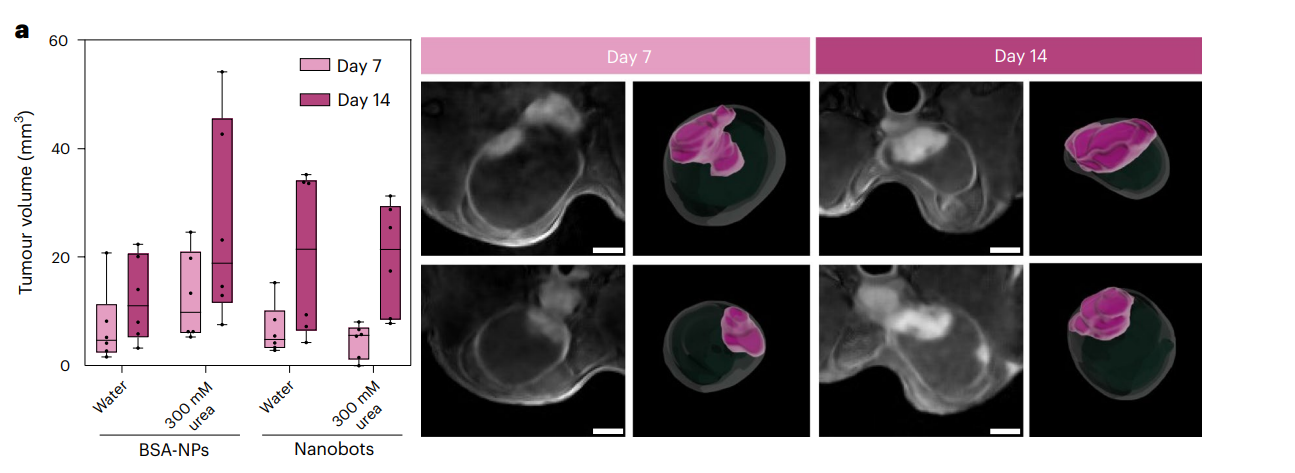
Figure 4. a, Left: tumour volumes (determined by MRI) on days 7 and 14 after cell implantation for the different study groups (n = 6 per group, biological replicates). Results are expressed in a box plot (centre line at the median; upper and lower bounds at 75th and 25th percentiles, respectively; one dot per animal) with whiskers at minimum and maximum values. Right: 2D DW-MRI images of the bladder (hypointense circular region) of two representative mice at t = 7 and 14 days after inoculation of MB49 cells. Scale bars, 2 mm. 3D renders of whole bladders (transparent) and tumours (purple) are presented next to each MRI image.
Significance of the Result:
The orthotopic model provided a relevant platform for testing the efficacy of urease-powered nanobots in a realistic biological context, reflecting human bladder cancer scenarios.
Key Innovations:
This model allowed for the direct assessment of nanobot accumulation and therapeutic efficacy in a biologically representative environment, essential for translating findings to clinical applications.
4. In Vivo Studies and Nanobot Administration
Key Steps:
- Mice with established tumors were administered radiolabeled urease-powered nanobots intravesically, with groups receiving either 18F-labeled nanobots in water or in 300 mM urea.
- Post-administration, PET-CT imaging was performed 3 hours later to assess nanobot accumulation.
Result and Key Data:
PET imaging revealed an eightfold increase in the accumulation of nanobots at the tumor site in the urea group compared to controls, with average tumor uptake reaching approximately 2.5% of the injected dose.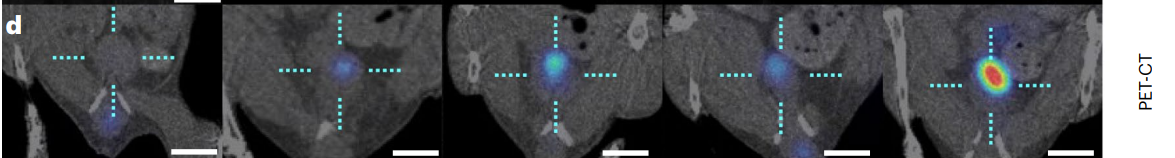
Figure 5. Coronal PET 2D images overlaid on CT images of one representative animal per study group. The dotted cyan cross shows the bladder position and the radioactive intensity has been colour-coded (given as the percentage of injected dose per millilitre, %ID cm−3 ). Scale bars, 5 mm.
Significance of the Result:
This dramatic increase in tumor localization highlights the potential of urease-powered nanobots to effectively deliver therapeutic agents directly to bladder tumors, addressing the limitations of traditional drug delivery methods.
Key Innovations:
The use of active propulsion via urease significantly improved targeting efficiency, marking a pivotal advancement in the field of nanomedicine for bladder cancer therapy.
5. Assessment of Therapeutic Efficacy through Radionuclide Therapy
Key Steps:
- Following imaging studies, mice were treated with either high or low doses of 131I-labeled nanobots in urea or water.
- Tumor sizes were measured pre- and post-treatment using MRI to evaluate therapeutic outcomes.
Result and Key Data:
The administration of high-dose 131I-nanobots in urea resulted in nearly 90% reduction in tumor size. In contrast, low doses still produced a halting of tumor growth, demonstrating significant therapeutic potential.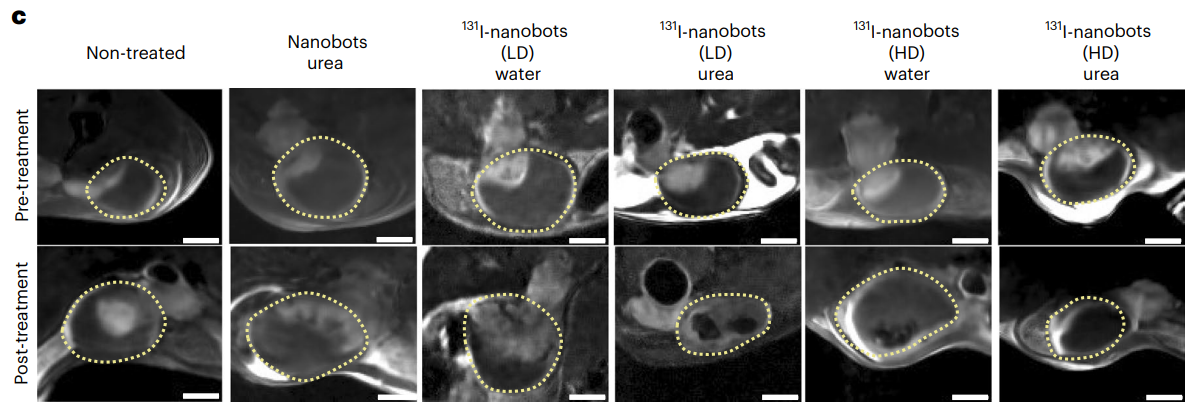
Figure 6. DW-MRI 2D slices through the bladder of tumour-bearing mice before and after treatment with radionuclides; low-dose (LD) and high-dose (HD) denote 1.85 MBq and 18.5 MBq doses of 131I, respectively; yellow dotted lines show the bladders. Scale bars, 2 mm.
Significance of the Result:
These findings suggest that urease-powered nanobots can deliver effective radionuclide therapy at lower doses, minimizing potential side effects and enhancing patient safety.
Key Innovations:
The ability of nanobots to achieve such therapeutic effects at lower doses of radionuclides represents a transformative approach to cancer treatment, maximizing efficacy while reducing toxicity.
6. Ex Vivo Imaging and Localization
Key Steps:
- Following treatment, bladders were harvested, cleared, and imaged using polarization-dependent scattered light-sheet microscopy (sLS) to assess nanobot localization within tumor tissues.
- The imaging technique allowed for 3D visualization of nanobot penetration into tumor regions.
Result and Key Data:
Analysis showed that nanobots accumulated significantly at the tumor surface, with penetration depth decreasing with distance from the surface. Quantification indicated that nanobot presence was about four times higher in tumors compared to healthy tissue.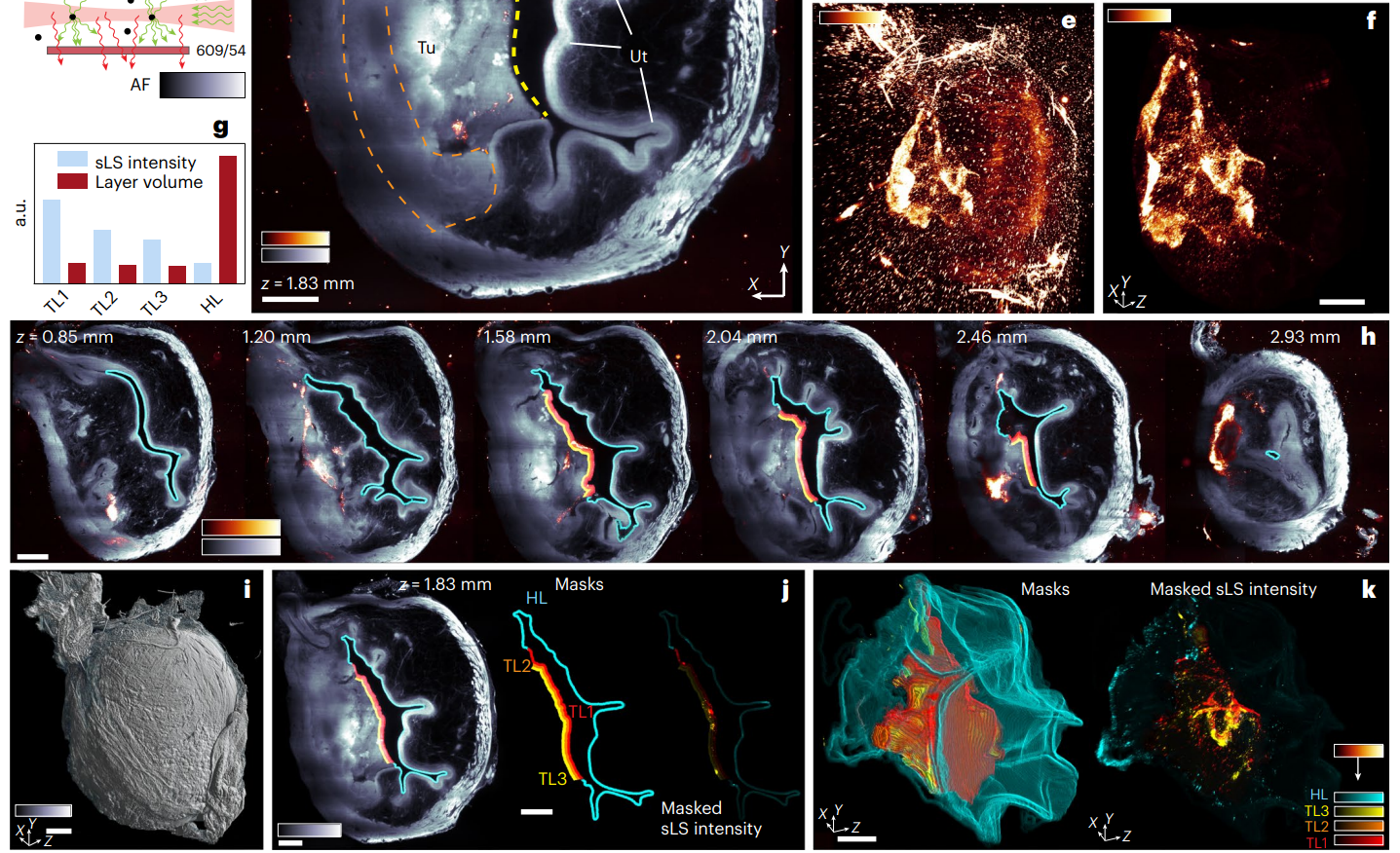
Figure 7. f, MIP of scattered signal from volume shown in d, containing only nanobots. g, Integrated sLS signal intensity normalized by layer volume inside four masks shown in h,j,k (Supplementary Methods).
Significance of the Result:
This step provided critical insights into the distribution and localization of nanobots, confirming their ability to penetrate tumor tissues effectively, which is crucial for therapeutic success.
Key Innovations:
The use of sLS for imaging offered a novel, label-free approach to visualize nanobot behavior in complex biological tissues, enhancing the understanding of their therapeutic delivery mechanisms
Conclusion
The findings of this study demonstrate that urease-powered nanobots significantly enhance the accumulation and penetration of therapeutic agents in bladder tumors, achieving a remarkable approximately 90% reduction in tumor size with intravesically administered radionuclide therapy. This innovative approach offers promising implications for the future of bladder cancer treatment, potentially increasing the efficacy of therapies that currently have limited effectiveness. However, the research acknowledges certain limitations, such as the need for further studies to assess long-term safety and efficacy in human models. Future research should focus on optimizing the nanobot design and exploring additional applications of this technology in other cancer types.
The introduction of urease-powered nanobots represents a significant leap forward in addressing the challenges of bladder cancer treatment, paving the way for more effective and targeted therapeutic strategies.
Reference:
Simó, Cristina, et al. “Urease-powered nanobots for radionuclide bladder cancer therapy.” Nature Nanotechnology 19.4 (2024): 554-564.
