A study has unveiled a novel chemoimmunotherapy strategy that employs engineered tumor-derived extracellular vesicles to enhance drug delivery and improve treatment outcomes for triple-negative breast cancer (TNBC).
Key Highlights
- Research Question:
How can the therapeutic efficacy of chemotherapy and immunotherapy be combined to enhance treatment outcomes in triple-negative breast cancer (TNBC)? - Research Difficulties:
Achieving effective cancer-homing delivery of therapeutics and optimizing the administration intervals for combined treatments remain significant challenges in TNBC therapy. - Key Findings:
The study demonstrated that using CRISPR/Cas9-edited tumor-derived extracellular vesicles significantly improved the encapsulation and delivery of doxorubicin, resulting in increased tumor destruction and enhanced immune response. - Innovative Aspects:
The research introduced a tandem-augmented approach utilizing tumor-derived extracellular vesicles (TDEVs) combined with a sequential treatment regimen to optimize therapeutic effects against TNBC. - Importance of the Study:
This study provides crucial insights into enhancing the effectiveness of chemoimmunotherapy for TNBC, potentially leading to improved clinical outcomes for patients suffering from this aggressive form of breast cancer.
Challenges and Opportunities in Treating Triple-Negative Breast Cancer
Triple-negative breast cancer (TNBC) is a particularly aggressive subtype of breast cancer, accounting for approximately 10-20% of all breast cancer diagnoses. It is defined by the lack of estrogen receptors, progesterone receptors, and human epidermal growth factor receptor 2 (HER2), making it resistant to conventional hormonal therapies and targeted treatments. The rapid progression and high recurrence rates associated with TNBC contribute to a dismal prognosis, with median overall survival rates ranging from 8 to 13 months. Current treatment strategies primarily involve chemotherapy, with anthracycline doxorubicin as a standard first-line option. However, the efficacy of these treatments is often hampered by severe side effects, tumor heterogeneity, and the development of chemoresistance, underscoring the urgent need for innovative therapeutic approaches that enhance treatment efficacy and improve patient outcomes.
Innovative Chemoimmunotherapy Strategy and Specific Research Objectives
The primary aim of this research is to enhance the therapeutic efficacy of combined chemotherapy and immunotherapy for patients with triple-negative breast cancer (TNBC). Specifically, this study seeks to address two critical challenges in TNBC treatment: the efficient delivery of therapeutic agents directly to tumor sites and the optimization of treatment intervals for combined therapies. The research objectives include:
- Develop a novel drug delivery system using CRISPR/Cas9-engineered tumor-derived extracellular vesicles (TDEVs) for targeted encapsulation and release of doxorubicin (DOX) within the tumor microenvironment.
- Investigate the impact of a tandem-augmented chemoimmunotherapy strategy by administering TDEVs carrying DOX in conjunction with immune checkpoint inhibitors (ICIs) at optimized intervals.
- Assess the therapeutic efficacy and safety of the combined treatment regimen in preclinical models, focusing on tumor growth suppression and immune response enhancement.
- Analyze the pharmacological properties of the engineered TDEVs and their ability to improve treatment outcomes in triple-negative breast cancer (TNBC) patients.
- Explore the potential for improved clinical application of the proposed treatment strategies for TNBC.
Experimental Design and Key Findings
Experimental Process Outline
- Collection of clinical tumor samples from TNBC patients before and after AC (doxorubicin plus cyclophosphamide) treatment.
- Analysis of patient samples through immunohistochemistry (IHC) and immunofluorescence (IF) staining to detect immune cell infiltration and PD-L1 expression.
- CRISPR/Cas9 gene editing to create PD-L1 knockout (Pd-l1KO) in 4T1 murine breast cancer cells.
- Isolation of tumor-derived extracellular vesicles (TDEVs) from 4T1 Pd-l1KO cells using differential centrifugation.
- Preparation of TDEV-fusogenic anthracycline doxorubicin liposomes (T-DOX) via membrane extrusion method.
- In vitro evaluation of T-DOX for cellular uptake and cytotoxicity against 4T1 cells.
- Induction of PD-L1 expression in 4T1 cells post-treatment with T-DOX.
- Immunogenic cell death (ICD) assessment through HMGB1 release and CRT exposure assays.
- In vivo studies on orthotopic 4T1 tumor-bearing mouse models to assess tumor targeting and therapeutic efficacy of T-DOX and S-PDNGs.
- Evaluation of safety and biodistribution of the treatments through organ histology and blood chemistry analysis.
Key Experiments Introduction
1. Analysis of Clinical Tumor Samples
Procedure: Clinical tumor samples from TNBC patients were examined using immunohistochemistry (IHC) and immunofluorescence (IF) staining for CD8+ T cells, Tregs, and PD-L1 expression after four courses of AC treatment.
Result: The analysis revealed profound CD8+ T cell infiltration in the tumors post-DOX treatment, with corresponding decreases in Foxp3+ Tregs. A trend toward increased PD-L1 expression was also observed in the treated tumor samples compared to untreated ones.
New Finding: These findings suggest that DOX treatment may help reverse the immunosuppressive tumor microenvironment and enhance PD-L1 expression, thereby supporting the potential for combining ICIs with DOX-based chemotherapy.
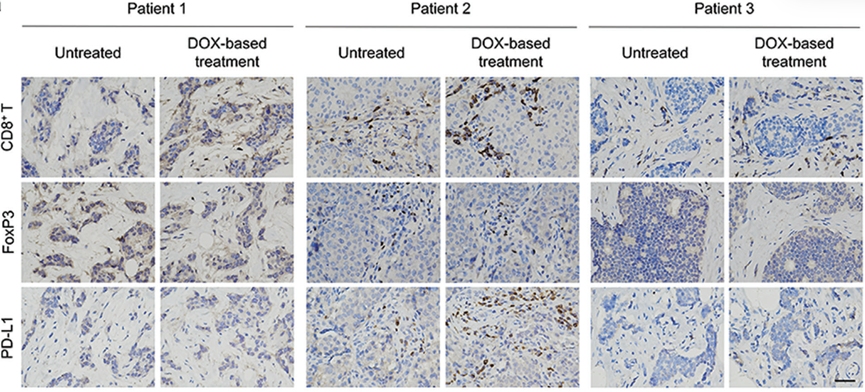
Figure 1. Representative immunohistochemistry (IHC) images analyzing CD8+, Foxp3+, and PD-L1 of tumor tissues from patients with TNBC.
2. Development of T-DOX
Procedure: TDEVs were isolated from 4T1 Pd-l1KO cells and co-extruded with doxorubicin liposomes to create T-DOX, achieving a maximum encapsulation efficiency of 97% at a lipid:TDEVs protein ratio of 1:0.2.
Result: The T-DOX displayed a spherical vesicular structure with an average size of approximately 120 nm and a sustained release profile of doxorubicin under physiological conditions (pH 7.4).
New Finding: The T-DOX demonstrated not only high drug encapsulation but also significant cancer-homing ability, enhancing its potential as an effective delivery system for targeted chemotherapy in TNBC.
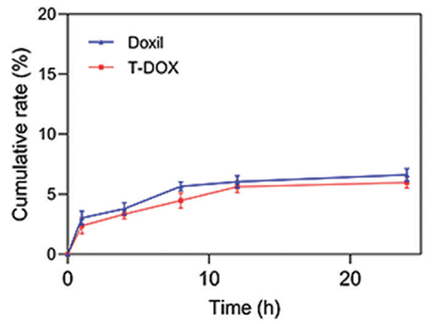
Figure 2. In vitro release profile of Doxil and T-DOX in PBS (pH 7.4).
3. In Vivo Efficacy of T-DOX and S-PDNGs
Procedure: In an orthotopic 4T1 tumor-bearing mouse model, mice were administered T-DOX followed by S-PDNGs at a 1-day interval. Tumor growth was monitored using bioluminescence imaging.
Result: The combination treatment resulted in a tumor inhibition rate of 76%, significantly higher than T-DOX (54%) and Doxil (42%).
New Finding: The enhanced tumor suppression observed with the sequential administration of T-DOX and S-PDNGs indicates a synergistic effect that could improve therapeutic outcomes for patients with TNBC.
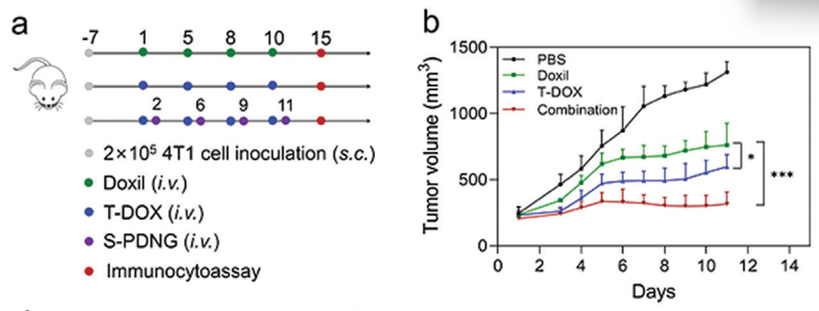
Figure 3. (a) Orthotopic 4T1 tumor-bearing mice (n = 5) were i.v. injected with PBS, Doxil, T-DOX, and combination therapy (T-DOX plus S-PDNGs) for a total of four administrations. (b) 4T1 tumor growth curve.
Implications and Future Directions for TNBC Treatment
In conclusion, the study demonstrates that the CRISPR/Cas9-edited TDEV-based biohybrid system for DOX delivery effectively induces immunogenic cell death (ICD) and upregulates PD-L1 expression in tumors, enhancing the overall immunotherapy response in TNBC. The research emphasizes that the optimized ordinal-interval regimen of S-PDNGs significantly contributes to reversing the immunosuppressive microenvironment, thereby increasing the effectiveness of cancer immunotherapy. Given the inherent resistance of TNBC to conventional treatment modalities, the proposed chemoimmunotherapy strategy is positioned as a promising clinical solution that could greatly benefit TNBC patients. The findings not only advance the understanding of TNBC treatment but also have the potential to inform therapeutic strategies for other malignancies characterized by similar immunosuppressive conditions.
Reference:
Sun, Mengchi, et al. “Immunogenic Nanovesicle‐Tandem‐Augmented Chemoimmunotherapy via Efficient Cancer‐Homing Delivery and Optimized Ordinal‐Interval Regime.” Advanced Science 10.1 (2023): 2205247.
