Editor: Nina
This study investigates the development of multifunctional nanoparticles for enhancing the effectiveness of chemo-photothermal cancer therapy through targeted drug delivery and dual-responsive behavior.
Key Preview
Research Question:
The study investigates how multifunctional nanoparticles can improve the effectiveness of chemo-photothermal cancer therapy by addressing challenges associated with targeted drug delivery and tumor treatment.
Research Design and Strategy:
The study develops hybrid nanoparticles by combining amorphous calcium carbonate (ACC) with polymeric components to create nanoparticles capable of targeted drug delivery and enhanced therapeutic effects. These nanoparticles are designed to be dual-responsive, reacting to both pH and reduction conditions in the tumor microenvironment.
Method:
A series of nanoparticles were synthesized using a gas diffusion reaction to create ACC nanoparticles and a film hydration method to encapsulate the chemotherapeutic agent doxorubicin (DOX) along with the photosensitizer indocyanine green (ICG). These nanoparticles were characterized using Fourier-transform infrared spectroscopy (FTIR), dynamic light scattering (DLS), transmission electron microscopy (TEM), and differential scanning calorimetry (DSC) to evaluate their structure and drug-loading capacity.
Key Results:
The synthesized nanoparticles demonstrated enhanced cellular uptake and exhibited an improved photothermal effect when exposed to near-infrared (NIR) laser irradiation. Additionally, the nanoparticles showed superior in vivo antitumor efficacy in 4T1 breast cancer-bearing mice, suggesting their potential as an effective treatment for cancer.
Significance of the Research:
The research offers valuable insights into the use of dual-responsive nanoparticles for chemo-photothermal therapy, potentially paving the way for more effective and targeted treatments for cancer. By combining the benefits of chemotherapy and photothermal therapy, these nanoparticles could minimize side effects while improving treatment outcomes.
Introduction
Evolution of Research:
Nanoparticle-based drug delivery systems have undergone significant advancement, evolving from simple carriers to complex multifunctional platforms that combine different therapeutic modalities, such as chemotherapy and photothermal therapy, to treat cancer more effectively. This evolution is driven by the need for more precise and controlled treatment approaches that minimize side effects and maximize therapeutic efficacy.
Limitations of Current Treatment:
Current cancer treatments often rely on single modalities, such as chemotherapy or photothermal therapy, which may be limited in their effectiveness and often lead to undesirable side effects. The challenge remains to combine these therapies in a single system that can target tumors more efficiently, overcome the limitations of individual therapies, and enhance overall treatment outcomes.
Research Question or Challenge:
This study addresses the challenge of integrating chemotherapy and photothermal therapy into a single nanoparticle system, aiming to enhance tumor targeting and improve treatment efficacy while reducing side effects. By utilizing dual-responsive nanoparticles that can respond to the acidic and reductive conditions of the tumor microenvironment, this research offers a promising solution to improve the effectiveness of cancer treatment.
Research Team and Objective
Research Team:
The research was conducted by Jingmou Yu, Liangliang Wang, Xin Xie, and others at Jiujiang University. Their findings were published in the International Journal of Nanomedicine in 2023.
Objective:
The primary objective of this research is to develop multifunctional nanoparticles that can efficiently and selectively deliver both chemotherapy (DOX) and photothermal therapy agents (ICG) to cancerous tissues. The aim is to enhance the effectiveness of cancer treatment by combining these therapeutic modalities in a single nanoparticle platform.
Innovations:
The key innovation of this research lies in the creation of dual-responsive nanoparticles that combine amorphous calcium carbonate (ACC) with polymeric materials, creating a system that can simultaneously deliver chemotherapy and photothermal therapy agents. This combination allows for targeted therapy with reduced side effects, making it a promising new approach in cancer treatment.
Experimental Process
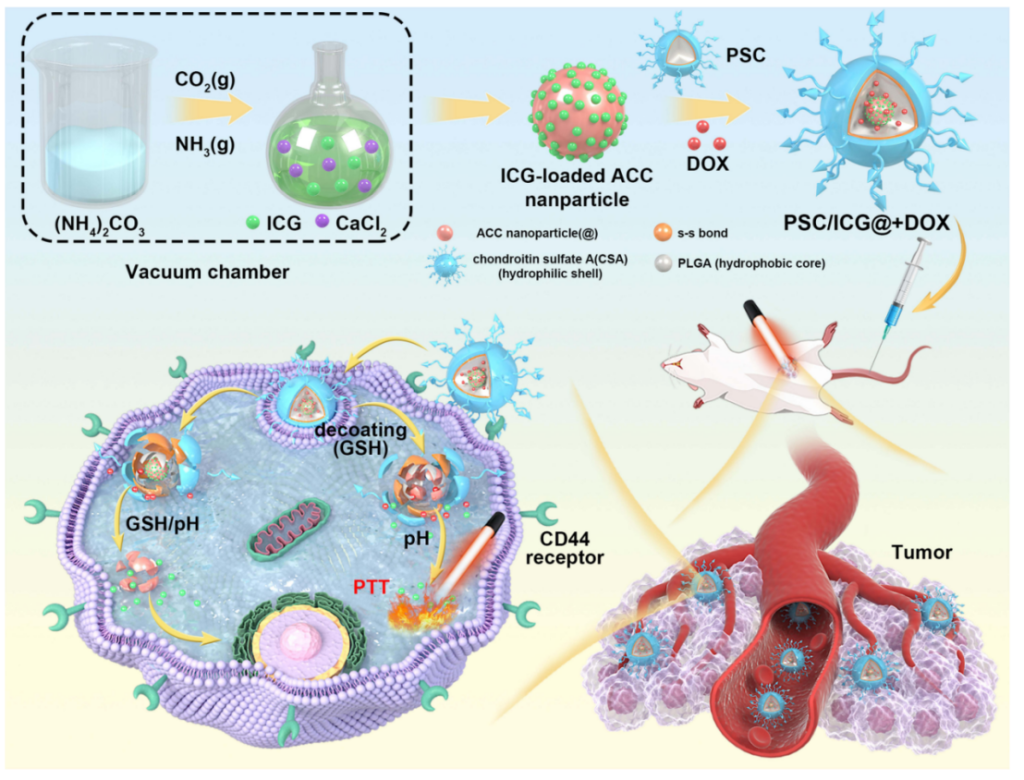
1. Preparation of ICG-loaded Amorphous Calcium Carbonate (ICG@) Nanoparticles
Key Steps:
- Calcium chloride (CaCl2) was dissolved in absolute ethanol to form a solution.
- Ammonium carbonate ((NH4)2CO3) was added into a separate beaker containing ethanol and kept in a vacuum desiccator at room temperature. The gas diffusion reaction took place over 18 hours, allowing the formation of amorphous calcium carbonate (ACC).
- Indocyanine green (ICG), a near-infrared (NIR)-absorbing dye used for photothermal therapy, was incorporated into the ACC nanoparticles by adding it to the CaCl2 solution during the preparation of the ACC.
Results and Key Data:
- The particle size of ICG-loaded ACC nanoparticles (ICG@) was found to be around 221 nm, measured by dynamic light scattering (DLS). This size is optimal for cellular uptake and avoiding rapid clearance from the bloodstream.
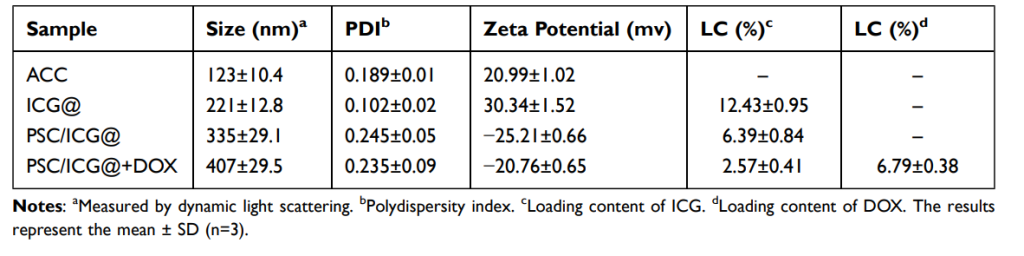
Table 1. Physicochemical Properties of Drug-Free and Drug-Loaded Nanoparticles
- Fourier-transform infrared spectroscopy (FTIR) confirmed the successful incorporation of ICG into the ACC nanoparticles by showing characteristic peaks from both ICG and ACC.
Significance of the Result:
- The successful incorporation of ICG into ACC nanoparticles is crucial for the photothermal properties required in cancer therapy. The ICG-loaded ACC nanoparticles offer a stable delivery platform for ICG, preventing its rapid degradation in aqueous environments and enhancing its effectiveness under NIR irradiation.
Key Innovations:
- The use of ACC nanoparticles as a carrier for ICG is innovative because ACC, being biodegradable, allows for controlled drug release in an acidic environment, which is a typical feature of the tumor microenvironment. The incorporation of ICG into ACC also enhances the stability of ICG, making it more suitable for therapeutic applications.
2. Encapsulation of DOX and ICG@ into Polymeric Nanoparticles (PSC/ICG@+DOX)
Key Steps:
- A polymeric nanoparticle system was developed using poly(lactic-co-glycolic acid) (PLGA) and chondroitin sulfate A (CSA) to form a core-shell structure.
- The PSC conjugate was synthesized by reacting PLGA with cystamine dihydrochloride to form a disulfide bond, which is redox-sensitive.
- DOX (doxorubicin) and ICG@ were encapsulated into the PSC nanoparticles via the film hydration method. The PSC conjugate was dissolved in a mixture of water and acetone, and ICG@ was dispersed in ethanol. The two solutions were combined, and the resulting mixture was stirred for 6 hours, followed by rotary vacuum evaporation and lyophilization.
Results and Key Data:
- The resulting PSC/ICG@+DOX nanoparticles were characterized by DLS, revealing an average size of 407 nm, larger than ICG@ alone, due to the additional polymer coating. The zeta potential was measured at -20.76 mV, indicating good stability of the nanoparticles.
- FTIR analysis confirmed the encapsulation of both ICG and DOX within the PSC nanoparticles, with clear shifts in the characteristic absorption peaks corresponding to DOX and ICG.
Significance of the Result:
- This step is crucial for creating a multifunctional drug delivery system that combines both chemotherapy (DOX) and photothermal therapy (ICG) into a single nanoparticle platform. The successful encapsulation of DOX and ICG in PSC nanoparticles allows for controlled and simultaneous delivery of both therapeutic agents to the tumor site.
Key Innovations:
- The use of disulfide-linked PSC conjugates offers a novel, redox-sensitive mechanism for drug release, which is triggered by the high levels of reduced glutathione (GSH) in the tumor microenvironment. This allows for site-specific release of DOX and ICG, minimizing toxicity to healthy tissues.
3. Characterization of PSC/ICG@+DOX Nanoparticles
Key Steps:
- Various techniques were employed to characterize the physical and chemical properties of the PSC/ICG@+DOX nanoparticles.
- FTIR spectroscopy was used to confirm the presence of ICG and DOX within the nanoparticles by analyzing the characteristic absorption peaks of both drugs.
- Differential scanning calorimetry (DSC) was used to determine the physical state of DOX and ICG within the nanoparticles. This helps identify whether the drugs are in an amorphous or crystalline form within the nanoparticle system.
- Transmission electron microscopy (TEM) was performed to observe the morphology of the nanoparticles.
Results and Key Data:
- FTIR confirmed the successful encapsulation of both ICG and DOX within the PSC nanoparticles, as evidenced by the distinct peaks of both drugs in the nanoparticle spectrum.
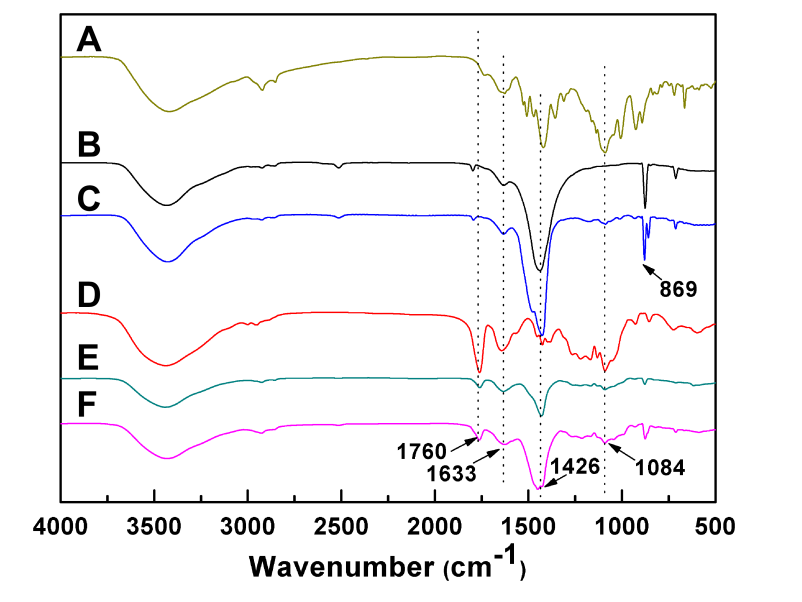
Figure 1. FTIR patterns of (A) ICG, (B) ACC, (C) ICG@, (D) PSC, (E) PSC/ICG@ and (F) PSC/ICG@+DOX nanoparticles.
- DSC analysis revealed that DOX and ICG were in an amorphous state within the nanoparticles, suggesting they were well-dispersed and not crystallized, which is beneficial for their solubility and controlled release.
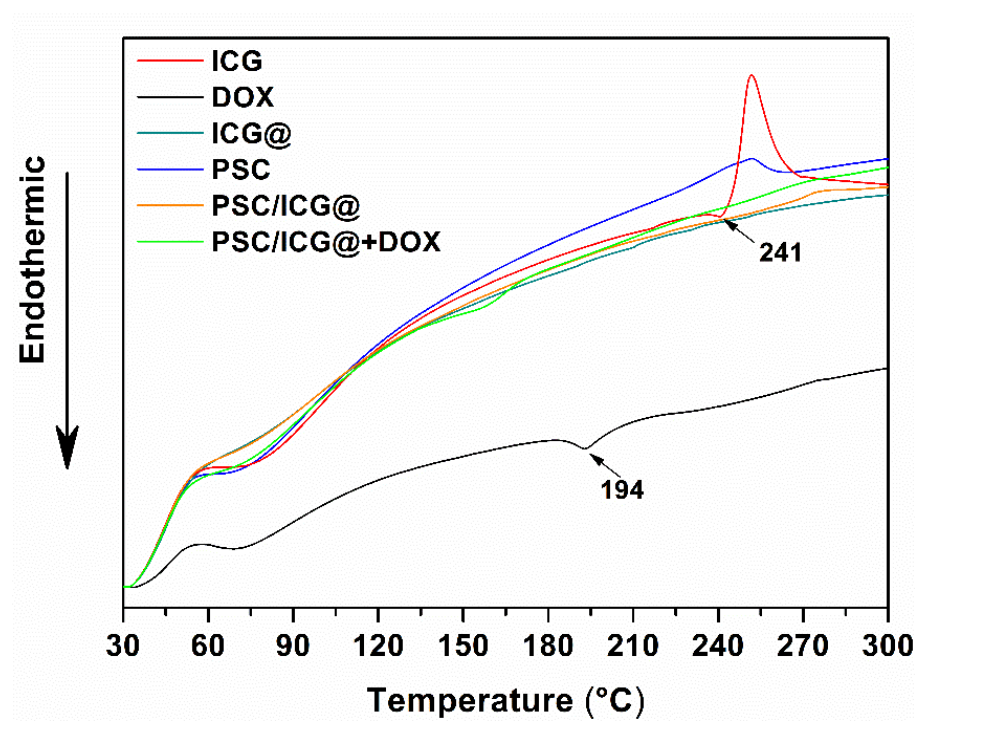
Figure 2. DSC thermograms of ICG, DOX, ICG@, PSC, PSC/ICG@ and PSC/ICG@+DOX nanoparticles.
- TEM images showed that the nanoparticles had a nearly spherical shape, with some aggregation observed for the ICG@ particles, which is typical for drug-loaded nanoparticles.
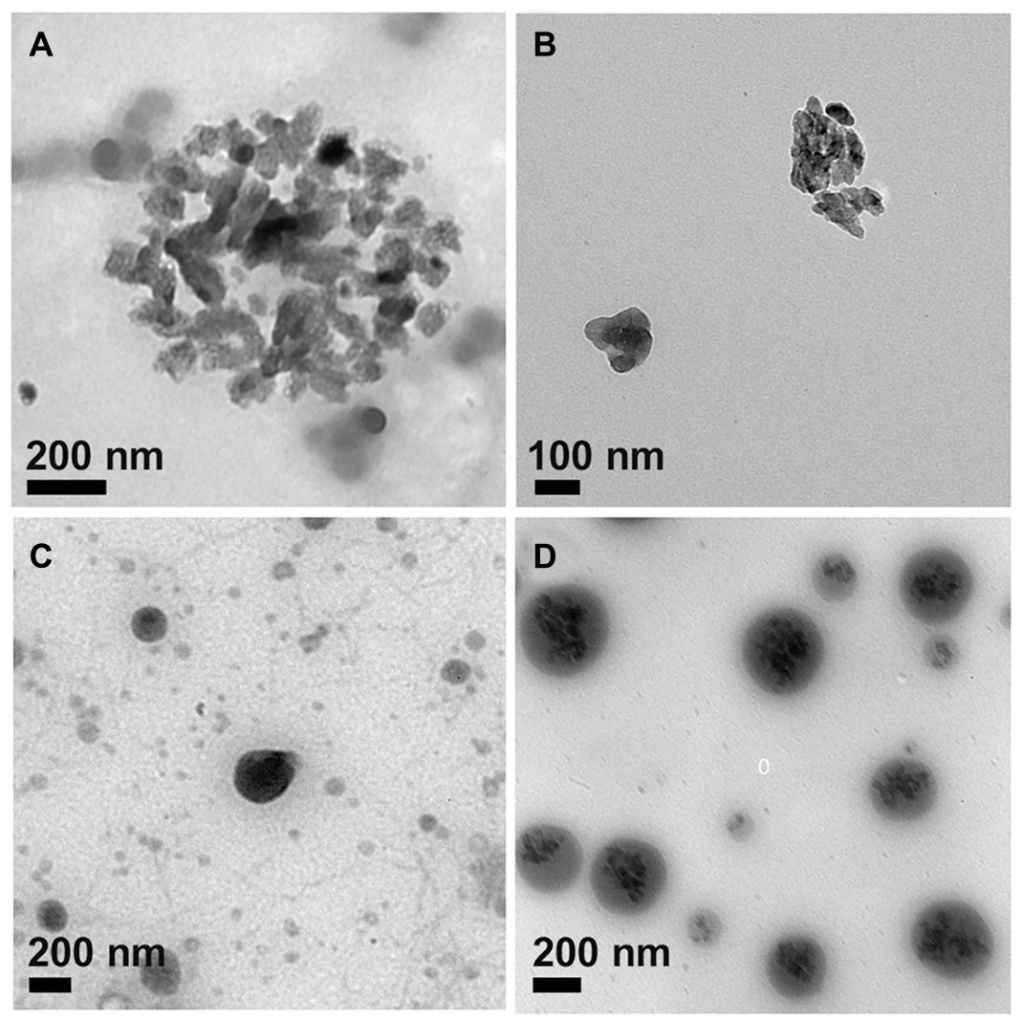
Figure 3. TEM images of (A) ACC, (B) ICG@, (C) PSC/ICG@ and (D) PSC/ICG@+DOX nanoparticles.
Significance of the Result:
- The results confirm that the PSC/ICG@+DOX nanoparticles have the desired structural characteristics and that the drugs are successfully encapsulated in a stable form. This is critical for ensuring the nanoparticles’ functionality in delivering the drugs in a controlled and effective manner.
Key Innovations:
- The use of multiple characterization techniques (FTIR, DSC, TEM) provides a comprehensive understanding of the nanoparticle structure and drug encapsulation efficiency, which is essential for the development of a reliable and reproducible drug delivery system.
4. In Vitro and In Vivo Testing: Drug Release, Cytotoxicity, and Antitumor Effects
Key Steps:
- In Vitro Drug Release: The release profiles of DOX from PSC/ICG@+DOX nanoparticles were studied using a dialysis method under different pH conditions (pH 7.4 and pH 5.5) to simulate the tumor microenvironment.
- Cytotoxicity Assays: The cytotoxicity of the nanoparticles was evaluated using an MTT assay in 4T1 breast cancer cells to assess their ability to kill cancer cells. The effect of NIR laser irradiation was also tested to evaluate the enhanced cytotoxicity from the photothermal effect.
- In Vivo Antitumor Effects: In vivo experiments were conducted using 4T1 tumor-bearing Balb/c mice. The antitumor efficacy was assessed by measuring tumor volume and performing histological analysis on tumor tissues.
Results and Key Data:
- In Vitro Drug Release: The PSC/ICG@+DOX nanoparticles exhibited pH-sensitive drug release. In acidic conditions (pH 5.5), the release of DOX was significantly higher than in neutral conditions (pH 7.4). The cumulative release of DOX at pH 5.5 with 20 mM GSH was 82.3%, demonstrating the nanoparticles’ responsiveness to both pH and reduction.
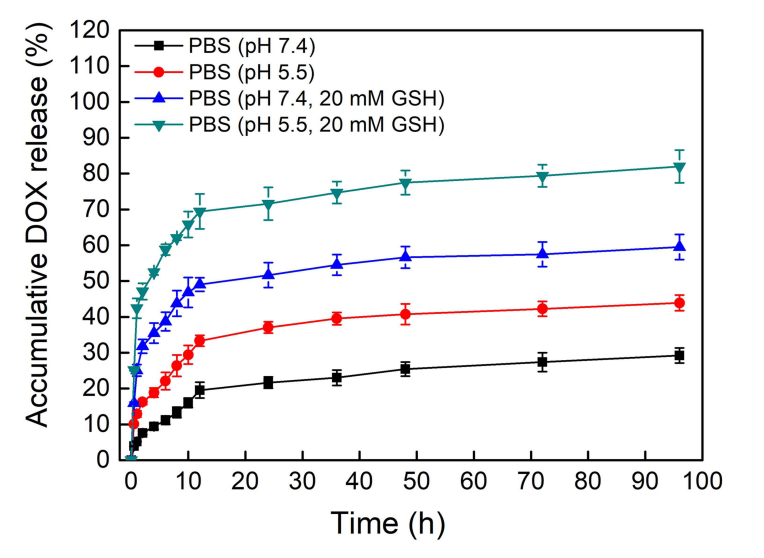
Figure 4. DOX release profiles of PSC/ICG@+DOX nanoparticles in PBS (pH 7.4, or pH 5.5) in the presence or absence of 20 mM GSH at 37°C.
- Cytotoxicity Assays: The nanoparticles showed with PSC/ICG@+DOX demonstrating the highest inhibition of cell viability in 4T1 cells, particularly when combined with NIR irradiation.
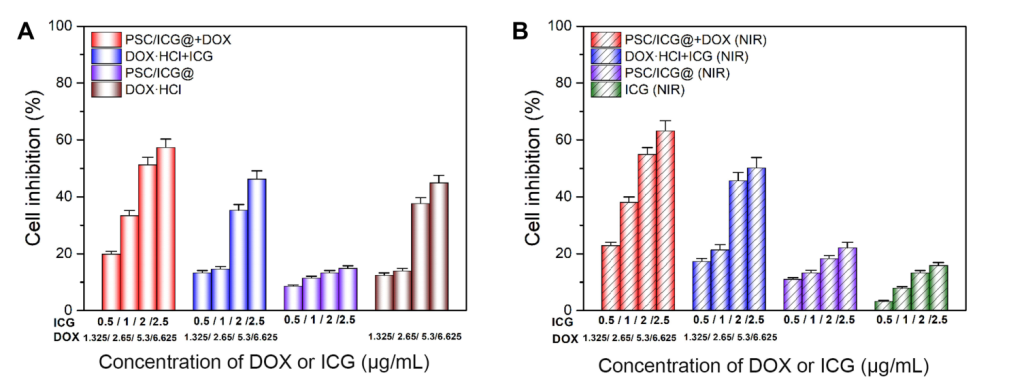
Figure 5. In vitro cytotoxicity in 4T1 cells treated with DOX·HCl, ICG, DOX·HCl+ICG, PSC/ICG@ nanoparticles, PSC/ICG@+DOX nanoparticles in the absence (A) or presence (B) of 808 nm NIR irradiation. (n=4)
- In Vivo Antitumor Effects: The nanoparticles exhibited superior antitumor effects in vivo, with the combination of chemotherapy and photothermal therapy (NIR irradiation) leading to the most significant tumor suppression, outperforming single therapies. Tumor growth was significantly reduced in the PSC/ICG@+DOX (NIR) group compared to other groups.
Significance of the Result:
- These results validate the potential of the PSC/ICG@+DOX nanoparticles for effective cancer treatment. The pH-sensitive and reduction-responsive release profiles, along with enhanced cytotoxicity under NIR irradiation, highlight the therapeutic advantages of using these nanoparticles in combination therapy. The in vivo data further demonstrate their efficacy in reducing tumor growth and enhancing therapeutic outcomes.
Key Innovations:
- The use of dual-responsive nanoparticles that release drugs in response to both the acidic pH and the high GSH concentration of the tumor microenvironment represents a significant innovation in targeted drug delivery. Furthermore, the combination of chemo-photothermal therapy within a single nanoparticle system is a novel approach to enhancing the effectiveness of cancer treatment while minimizing side effects.
Conclusion
Key Findings:
The study confirms that PSC/ICG@+DOX nanoparticles offer a promising strategy to enhance chemo-photothermal cancer therapy. The nanoparticles demonstrated improved tumor targeting, enhanced cellular uptake, and synergistic therapeutic effects when exposed to NIR irradiation, providing a more effective treatment option compared to chemotherapy or photothermal therapy alone.
Academic Contributions and Application Potential:
This research contributes to the field of cancer therapy by advancing nanoparticle technology, combining chemotherapy and photothermal therapy in a single system. This dual-modal approach has significant potential for clinical applications, offering a more effective and targeted cancer treatment option with fewer side effects.
Limitations:
The study’s limitations include the need for further research to evaluate the long-term biocompatibility of the nanoparticles and to optimize the formulation for large-scale production. The stability of the nanoparticles over extended periods and their behavior in clinical settings also require further investigation.
Future Research Suggestions:
Future studies could focus on optimizing the nanoparticle formulation for different types of cancer, exploring additional in vivo evaluations to assess safety and efficacy, and conducting clinical trials to further investigate the therapeutic potential of the dual-responsive nanoparticles in cancer treatment.
Reference
Yu, Jingmou, et al. “Multifunctional nanoparticles codelivering doxorubicin and amorphous calcium carbonate preloaded with indocyanine green for enhanced chemo-photothermal cancer therapy.” International Journal of Nanomedicine (2023): 323-337.
