Author: Tiffany
Researchers have developed a novel biomimetic hypoxia-triggered RNA interference (RNAi) nanomedicine that significantly enhances chemotherapy and radiotherapy efficacy for glioblastoma, a notoriously aggressive brain cancer.
Key Highlights
- Research Question:
Can RNA interference (RNAi) therapy effectively enhance the treatment outcomes of glioblastoma through innovative drug delivery systems? - Research Difficulties:
The main challenges included the efficient delivery of siRNA to glioblastoma cells and overcoming the tumor’s hypoxic environment, which complicates treatment. - Key Findings:
The study found that a cancer cell membrane-disguised RNAi nanomedicine improved blood circulation, targeted glioblastoma sites effectively, and enhanced chemotherapy and radiotherapy responses by silencing the phosphoglycerate kinase 1 (PGK1) gene. - Innovative Aspects:
This research introduces a biomimetic approach utilizing cancer cell membranes to improve drug delivery and targeting, coupled with a hypoxia-responsive release mechanism for enhanced therapeutic activity. - Importance of the Study:
The findings represent a significant advancement in glioblastoma treatment strategies, potentially leading to improved survival rates and therapy effectiveness for patients.
Understanding Glioblastoma: Challenges and Target Identification
Glioblastoma (GBM) is a primary malignant brain tumor characterized by its rapid growth and an aggressive nature, resulting in a dismal prognosis with a five-year survival rate of less than 10%. This formidable cancer frequently arises in the cerebral hemispheres, leading to a range of debilitating symptoms, including severe headaches, seizures, and cognitive impairments, which drastically affect patients’ quality of life. The standard treatment regimen typically involves a combination of maximal surgical resection, chemotherapy, and radiotherapy; however, these strategies often yield limited success due to the tumor’s inherent resistance mechanisms and ability to rapidly develop treatment resistance. Recent research has highlighted the role of specific gene mutations in GBM progression, emphasizing the need for innovative therapeutic approaches that can effectively target these mutations. Among these, phosphoglycerate kinase 1 (PGK1) has emerged as a critical player in cellular energy metabolism and tumor survival, making it a promising target for RNA interference (RNAi) strategies aimed at enhancing treatment efficacy.
Innovative Nanomedicine Development: Aim and Objectives
The primary aim of this research was to develop an innovative and effective RNA interference (RNAi) delivery system specifically designed to improve treatment outcomes for glioblastoma (GBM). This study sought to address the significant limitations associated with conventional RNAi therapies, particularly the challenges related to the efficient delivery of short interfering RNA (siRNA) to tumor cells and the need to overcome the tumor’s hypoxic microenvironment, which often hinders therapeutic efficacy.
To achieve this aim, the research focused on several key objectives:
- Develop a biomimetic, hypoxia-triggered RNA interference (RNAi) nanomedicine for targeted delivery of siRNA to glioblastoma (GBM) cells.
- Investigate the silencing of the phosphoglycerate kinase 1 (PGK1) gene to enhance the sensitivity of GBM cells to chemotherapy and radiotherapy.
- Evaluate the therapeutic efficacy of the RNAi nanomedicine in combination with standard treatment modalities, such as paclitaxel and ionizing radiation.
- Elucidate the mechanisms by which PGK1 silencing and hypoxia-responsive release improve treatment outcomes in GBM.
Experimental Design: Key Methodologies and Outcomes of the RNAi Nanomedicine
Experimental Process Outline
- Synthesis of amphiphilic poly(MIs)-PEI block copolymer.
- Preparation of cancer cell membrane (CCM) vesicles from U87MG cells.
- Formulation of poly(MIs)/PTX@PEI/siPGK1@CCM nanomedicine.
- Characterization of nanomedicine (size, morphology, zeta potential).
- Evaluation of siPGK1 loading and encapsulation efficiency.
- Investigation of hypoxia-triggered release behavior of siPGK1.
- In vitro BBB transcytosis using bEnd.3 brain endothelial cells.
- Cellular uptake studies using confocal laser scanning microscopy (CLSM).
- Assessment of PGK1 expression and silencing efficiency via Western blot analysis.
- In vitro cytotoxicity assays on U87MG cells.
- Colony formation assays post-radiation treatment.
- In vivo biodistribution studies in U87MG glioblastoma-bearing nude mice.
- Evaluation of in vivo antitumor efficacy using bioluminescence imaging.
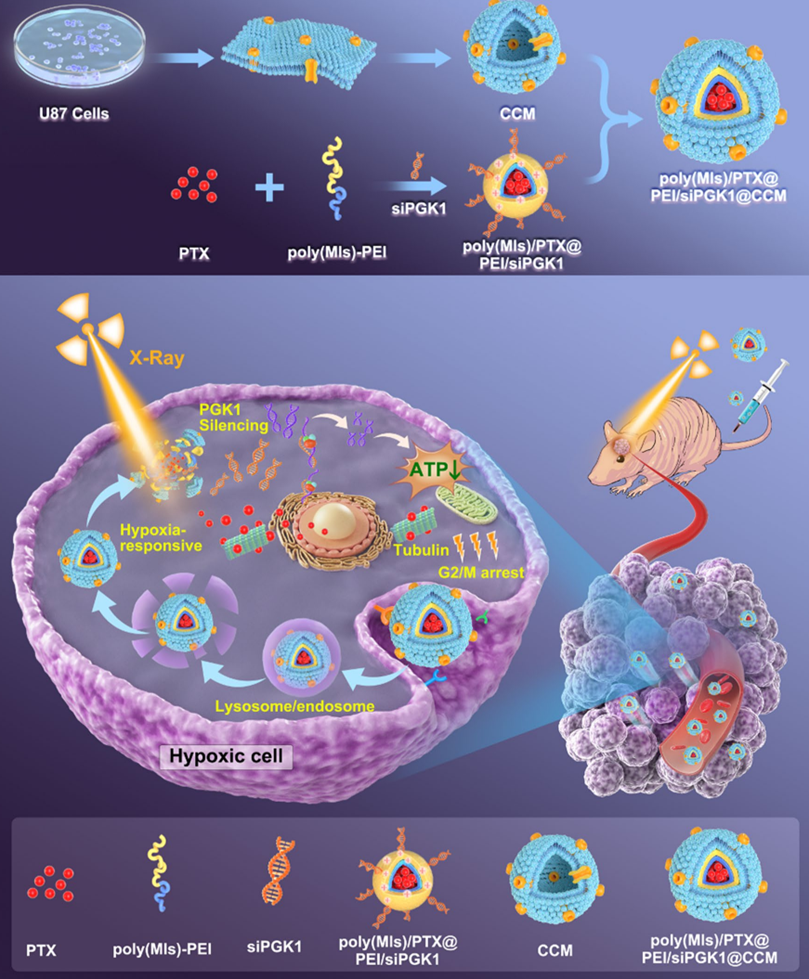
Figure 1. Graphical Abstract.
Key Experiments
1. Formulation of poly(MIs)/PTX@PEI/siPGK1@CCM Nanomedicine
- Procedure: The study involved dissolving 50 mg of poly(MIs)-PEI in DMSO and adding paclitaxel (PTX). Afterward, siPGK1 was condensed on the PEI backbone and coated with CCM via coextrusion.
- Result: The resulting nanomedicine exhibited a hydrodynamic diameter of approximately 107 nm with a zeta potential shift indicating successful CCM coating.
- New Finding: This formulation demonstrated significant stability and protective capabilities in the bloodstream, enhancing the delivery of siPGK1 to GBM cells.
2. In vitro BBB Transcytosis
- Procedure: The transwell model with bEnd.3 cells was employed to evaluate the transport of free FAM-siPGK1 and poly(MIs)/PTX@PEI/siPGK1@CCM across the blood-brain barrier (BBB) over various time points.
- Result: Poly(MIs)/PTX@PEI/siPGK1@CCM displayed a cumulative transport efficiency that was approximately 20-fold higher than that of free siPGK1.
- New Finding: This highlighted the enhanced ability of the biomimetic nanomedicine to cross the BBB effectively, a crucial factor in treating GBM.
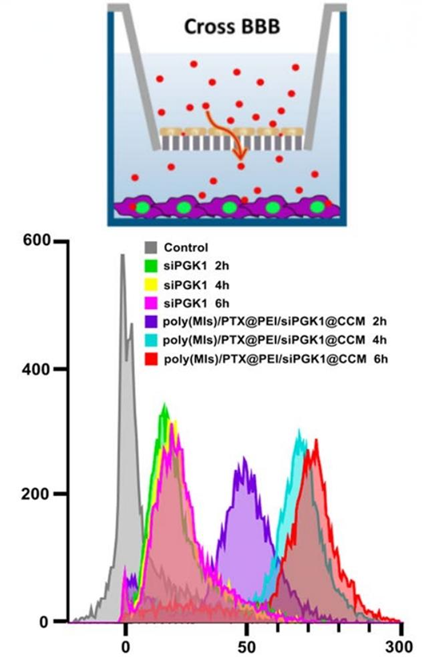
Figure 2. Cumulative transport of poly(MIs)/PTX@PEI/siPGK1@CCM at diferent time points across the bEnd.3 monolayer. Inset: schematic illustration of BBB model.
3. PGK1 Expression Silencing Efficiency
- Procedure: U87MG cells were treated with varying concentrations of poly(MIs)/PTX@PEI/siPGK1@CCM, and PGK1 protein levels were assessed through Western blot analysis after 24 hours.
- Result: The study found that PGK1 protein levels were downregulated by up to 83% at the maximum siRNA concentration of 300 × 10⁻⁹ M.
- New Finding: This significant reduction in PGK1 expression demonstrated the efficacy of the RNAi nanomedicine in targeting tumor metabolism.
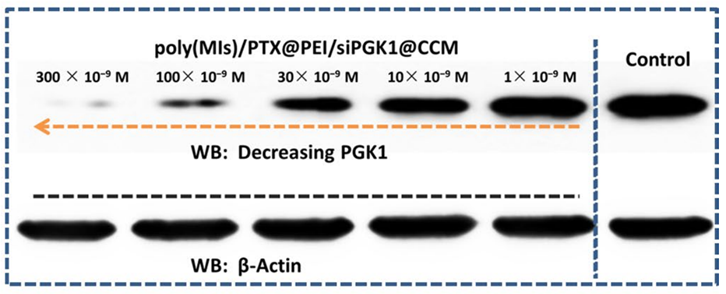
Figure 3. PGK1 protein inhibition capacity after 24 h of transportation.
4. In vivo Antitumor Efficacy
- Procedure: U87MG glioblastoma-bearing nude mice were treated with poly(MIs)/PTX@PEI/siPGK1@CCM + RT, and tumor growth was monitored using bioluminescence imaging.
- Result: Mice treated with the combination therapy exhibited a median survival time of 77.5 days, significantly longer compared to control groups.
- New Finding: This indicated that the synergistic effect of PGK1 silencing and radiotherapy drastically improved survival outcomes in an orthotopic GBM model.
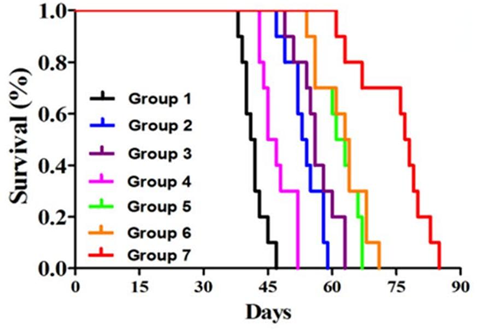
Figure 4. Kaplan–Meier survival curves for the mice (n=10) after diferent treatment strategies.
Findings and Implications: Enhancing Glioblastoma Treatment Efficacy
This study successfully developed a novel biomimetic hypoxia-triggered RNA interference (RNAi) nanomedicine, poly(MIs)/PTX@PEI/siPGK1@CCM, designed to enhance the therapeutic efficacy of chemotherapy and radiotherapy in treating glioblastoma (GBM). Key findings demonstrated that the nanomedicine not only prolonged blood circulation and effectively crossed the blood-brain barrier (BBB) but also targeted glioblastoma cells through homotypic recognition. The silencing of the phosphoglycerate kinase 1 (PGK1) gene was shown to sensitize GBM cells to treatment, leading to significant tumor suppression and improved survival rates in vivo. The innovative use of cancer cell membrane cloaking and a hypoxia-responsive release system represents a significant advancement in targeted cancer therapy.
The researchers emphasized that their synthesized RNAi nanomedicine exhibited superior glioblastoma inhibition in vitro and significantly prolonged survival in vivo without evident systemic immune clearance. They underscored the unique properties of the amphiphilic cationic block copolymer and its effectiveness in achieving targeted delivery and enhanced therapeutic response. However, a major limitation noted by the researchrs is the use of immune-deficient mice and cell line-derived GBM models, which may not fully represent the heterogeneity and complexity of human GBM. This limitation calls for further research, ideally involving patient-derived xenograft models, to validate the clinical applicability of their findings.
Reference:
Wang, Zhen, et al. “Biomimetic hypoxia-triggered RNAi nanomedicine for synergistically mediating chemo/radiotherapy of glioblastoma.” Journal of Nanobiotechnology 21.1 (2023): 210.
