Editor: Nina
The study demonstrates that Zerumbone-loaded liquid crystalline nanoparticles significantly enhance anti-cancer efficacy by inhibiting the proliferation and migration of non-small-cell lung cancer A549 cells, while modulating key tumor suppressor genes and proteins involved in cancer progression.
Key Preview
Research Question
The study investigates whether Zerumbone (ZER) loaded into liquid crystalline nanoparticles (LCNs) can effectively inhibit the proliferation and migration of non-small cell lung cancer (NSCLC) cells, specifically A549 cells.
Research Design and Strategy
The researchers designed an in vitro study to compare the efficacy of ZER incorporated into LCNs against free ZER. They utilized a series of assays to assess cell proliferation, migration, and underlying molecular mechanisms.
Method
The study involved synthesizing ZER-LCNs using the ultrasonication method, characterizing their physicochemical properties, and then testing their effects on A549 cells through various biological assays, including MTT, colony formation, wound healing, and transwell migration assays.
Key Results
The ZER-LCN formulation demonstrated significantly greater inhibition of A549 cell proliferation and migration compared to free ZER, even at lower concentrations. The study also established that ZER-LCNs upregulated tumor suppressor genes P53 and PTEN while downregulating the metastasis-associated gene KRT18.
Significance of the Research
This research suggests that the formulation of ZER into LCNs not only enhances its bioavailability but also improves its therapeutic efficacy against NSCLC, paving the way for potential clinical applications in cancer treatment.
Introduction
Lung cancer remains a leading cause of cancer-related mortality worldwide, with non-small-cell lung cancer (NSCLC) accounting for approximately 85% of all lung cancer cases. Traditional treatment options often suffer from limitations such as drug resistance and high toxicity. Recent advancements in nanotechnology have opened new avenues for drug delivery systems, particularly in enhancing the bioavailability of poorly soluble therapeutic agents. Zerumbone (ZER), a natural compound derived from the roots of Zingiber zerumbet, has shown promise as an anti-cancer agent but is hampered by low aqueous solubility and poor gastrointestinal absorption.
This study aims to address the challenges associated with ZER by formulating it into liquid crystalline nanoparticles (LCNs). The primary research question focuses on whether ZER-LCNs can effectively inhibit the proliferation and migration of A549 lung cancer cells compared to free ZER. By exploring this innovative approach, the research seeks to enhance the therapeutic potential of ZER, paving the way for more effective cancer treatments.
Research Team and Objective
The research team comprises Bikash Manandhar, Keshav Raj Paudel, and several collaborators, conducted this study at the University of Technology Sydney, Australia. The paper titled “Zerumbone-incorporated liquid crystalline nanoparticles inhibit proliferation and migration of non-small-cell lung cancer in vitro” was published in Naunyn-Schmiedeberg’s Archives of Pharmacology. The objective of this research is to develop a novel delivery system for ZER that enhances its anti-cancer activity and minimizes the adverse effects typically associated with conventional treatments for NSCLC.
Experimental Process
Experiment 1: Preparation of ZER-LCNs
Key Steps:
- Synthesis of ZER-LCNs:
- Monoolein (MO) was heated to 60 °C in a water bath.
- ZER was added to the molten MO and mixed until completely dissolved.
- P407 was heated and dissolved in water at 60 °C and added to the ZER-MO mixture.
- The combined mixture was ultrasonicated for 5 minutes, with cycles of 5 seconds on and 5 seconds off.
Results and Key Data:
- The resulting ZER-LCNs exhibited a mean particle size of 180.6 ± 3 nm, with a narrow polydispersity index (PDI < 0.4).
- The entrapment efficiency of ZER in the LCNs was high, reaching 90.63 ± 0.13%.
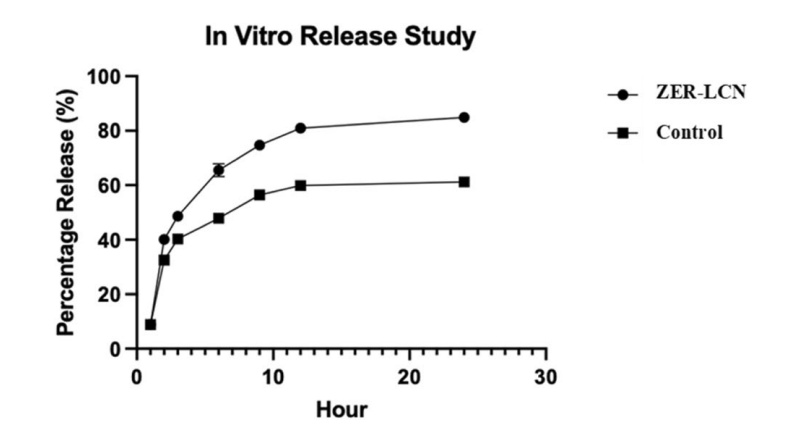
Figure 1.The in vitro release study of free ZER (control) and ZER-LCNs.
Significance of the Result:
- The successful formulation enhances the solubility and bioavailability of ZER, addressing its limitations.
Key Innovations:
- The ultrasonication method allowed for precise control over particle size and distribution, improving targeting and reducing side effects.
Experiment 2: In Vitro Release Studies
Key Steps:
- Setting Up Release Conditions:
- ZER-LCNs were placed inside dialysis tubing and submerged in PBS at pH 7.4.
- Samples were taken at predetermined intervals (1, 2, 3, 6, 9, 12, and 24 hours) to measure ZER release.
Results and Key Data:
- The cumulative release profile indicated that ZER-LCNs achieved over 80% drug release within 24 hours, significantly higher than the approximately 60% release from free ZER.
Significance of the Result:
- Sustained release suggests prolonged therapeutic effects, critical for effective cancer treatment.
Key Innovations:
- The use of dialysis membranes for evaluating drug release kinetics provides a reliable method to assess pharmacokinetic behavior.
Experiment 3: Cell Proliferation Assay
Key Steps:
- Conducting the MTT Assay:
- A549 cells were plated in 96-well plates and treated with varying concentrations of ZER-LCNs (1, 2.5, 5, 7.5, and 10 µM) and free ZER (1, 5, 10, 25, and 50 µM) for 24 hours.
- MTT reagent was added, and after a 4-hour incubation, formazan crystals were dissolved in DMSO.
- Absorbance was measured at 540 nm.
Results and Key Data:
- ZER-LCNs demonstrated an approximate 45% inhibition of A549 cell proliferation at a concentration of 5 µM, while free ZER showed minimal effects even at 50 µM.
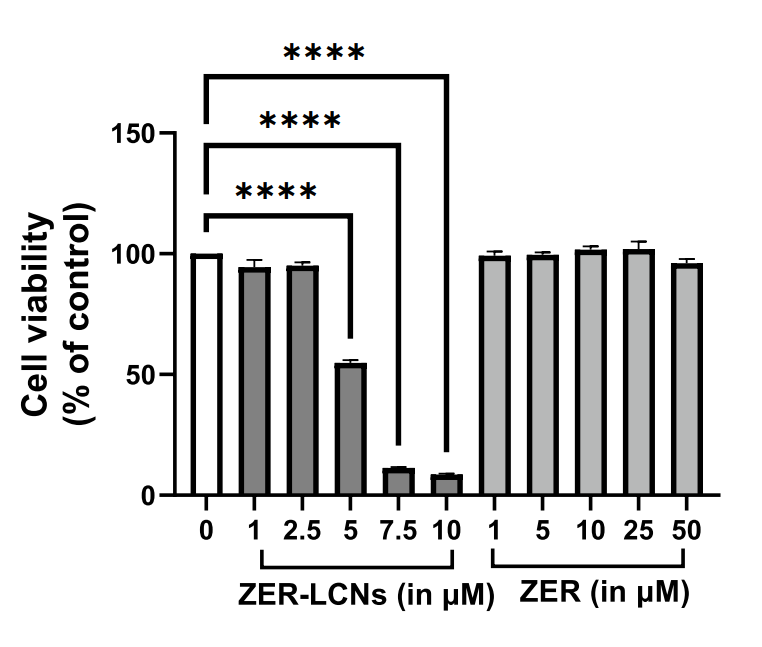
Figure 2. Anti-proliferative efects of ZER and ZER-LCNs in A549 cells. A549 cells were treated with or without ZER-LCNs (1, 2.5, 5, 7.5, or 10 µM) or ZER (1, 5, 10, 25, or 50 µM) for 24 h, followed by incubation with MTT. The purple formazan crystals formed were dissolved with DMSO and the absorbance was measured with micro-plate reader. The data in the fgure are mean ± SEM of 3 independent experiments. ****p<0.0001
Significance of the Result:
- Substantial inhibition of cell proliferation by ZER-LCNs highlights enhanced efficacy over free ZER.
Key Innovations:
- Achieving significant anti-proliferative effects at lower doses suggests a potential to minimize adverse side effects.
Experiment 4: Colony Formation Assay
Key Steps:
- Evaluating Colony Formation:
- A549 cells were seeded at a density of 500 cells per well in 6-well plates and treated with ZER (50 µM) or ZER-LCNs (5 µM) for two weeks.
- Cells were fixed with 3.7% formaldehyde and stained with crystal violet.
Results and Key Data:
- ZER-LCNs resulted in a significant reduction in colony formation compared to both control and ZER-treated groups.
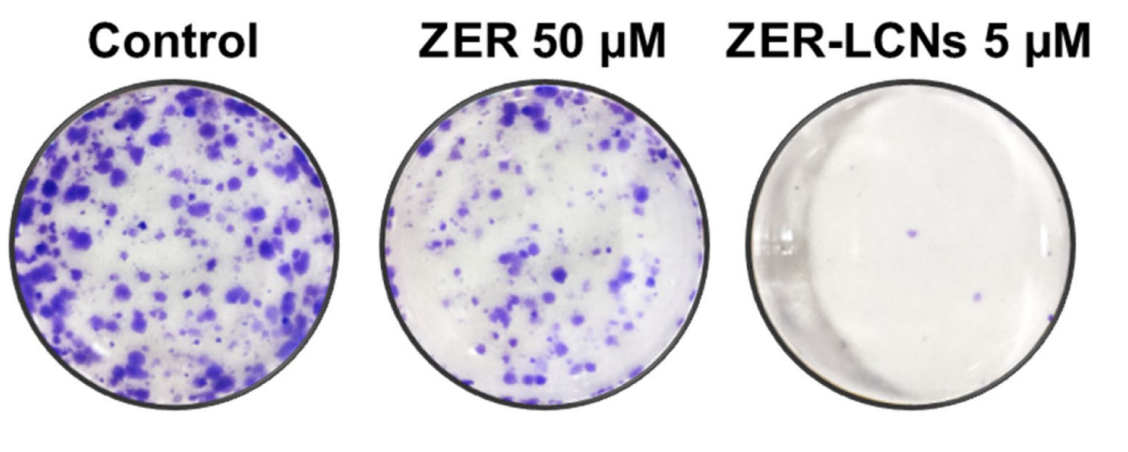
Figure 3. Efect of ZER and ZER-LCNs on colony formation of A549 cells. After seeding A549 cells in 6-well plate, they were cultured for 2 weeks in the absence or presence of 50 µM ZER or 5 µM ZER-LCNs. The cell colonies were stained with crystal violet and photographed. The fgure shows representa-tive images from 3 independent experiments
Significance of the Result:
- This assay demonstrates long-term anti-cancer effects, suggesting potential in preventing tumor regrowth.
Key Innovations:
- Combining colony formation assays with nanoparticle treatment provides insight into sustained effects on cancer growth.
Experiment 5: Wound Healing and Migration Assays
Key Steps:
- Wound Healing Assay:
- A549 cells were cultured to confluence in 6-well plates, and a scratch was made.
- Cells were treated with ZER (50 µM) or ZER-LCNs (2.5 and 5 µM) for 24 hours, and images were captured at 0 and 24 hours.
- Transwell Migration Assay:
- A549 cells were seeded in the upper chamber of a transwell plate and treated similarly.
- Migrated cells in the lower chamber were quantified after 24 hours.
Results and Key Data:
- ZER-LCNs significantly inhibited wound closure by ~27% at 5 µM, while free ZER did not show significant effects. In transwell assays, ZER-LCNs reduced cell migration by ~60%.
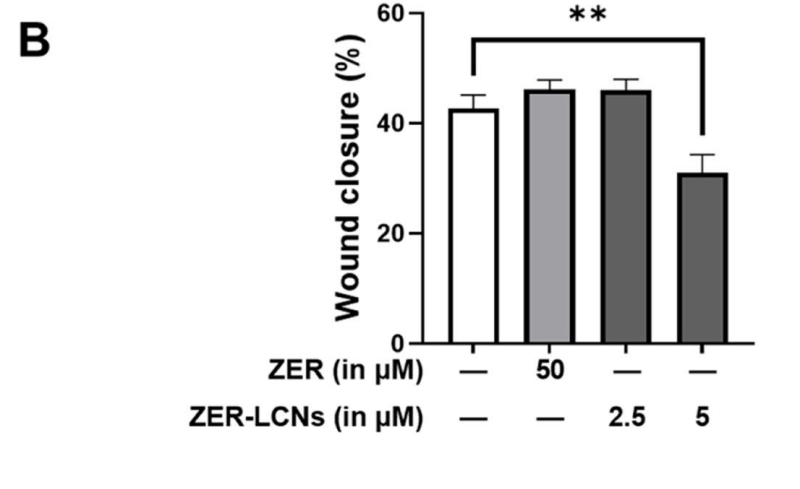
Figure 4. % wound closure after 24 h of incubation with or without ZER-LCNs. Data in B is expressed as mean ± SEM of 3 independent experiments. **p<0.01
Significance of the Result:
- The ability to inhibit cell migration highlights potential in preventing cancer metastasis.
Key Innovations:
- Using both wound healing and transwell assays offers a robust assessment of anti-migratory effects.
Experiment 6: Gene Expression Analysis
Key Steps:
- RT-qPCR for Gene Expression:
- A549 cells were treated with ZER (50 µM) or ZER-LCNs (2.5 and 5 µM) for 24 hours.
- Total RNA was extracted, and cDNA was synthesized. RT-qPCR was performed to analyze the expression levels of P53, PTEN, and KRT18.
Results and Key Data:
- Treatment with ZER-LCNs resulted in significant upregulation of P53 and PTEN mRNA levels and downregulation of KRT18.
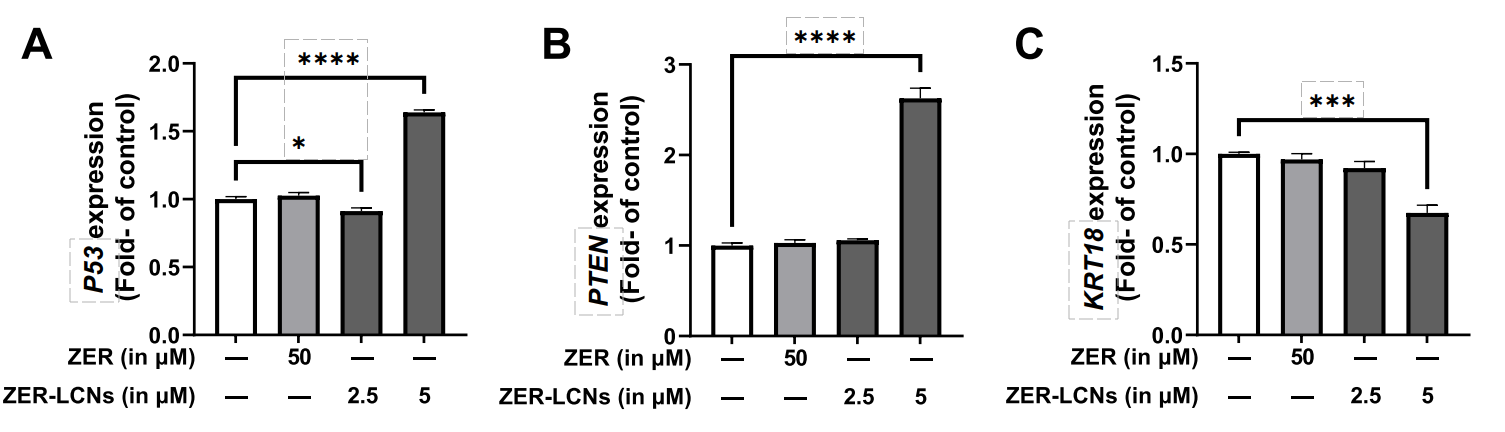
Figure 5. Regulation of mRNA levels P53, PTEN, and KRT18 by ZER and ZER-LCNs. A549 cells were treated with 50 µM ZER, 2.5 µM ZER-LCNs, or 5 µM ZER-LCNs for 24 h. Total RNA was extracted, cDNA synthesized, and mRNA levels were determined with qPCR. The fgure shows the mRNA levels of P53 (A), PTEN (B) and KRT18 ©, normalized against the levels of GAPDH. Data are expressed as mean ±SEM of 3 independent experiments. *p<0.05, ***p<0.001, ****p<0.0001
Significance of the Result:
- Modulation of these key tumor suppressor genes suggests that ZER-LCNs may influence critical pathways in cancer progression.
Key Innovations:
- Assessing gene expression changes in response to nanoparticle treatment provides insights into molecular mechanisms underlying anti-cancer effects.
Conclusion
The study concludes that ZER-LCNs exhibit significant anti-proliferative and anti-migratory effects on A549 cells, attributed to their ability to regulate the expression of key genes and proteins involved in cancer progression. The findings highlight the potential of using nanotechnology to enhance the therapeutic efficacy of ZER, offering a promising alternative for the treatment of NSCLC. However, the research is limited to in vitro studies, necessitating future investigations involving in vivo models to further validate the therapeutic potential of ZER-LCNs in clinical settings. Future research could also explore the formulation of ZER-LCNs for other cancer types and the optimization of delivery methods to maximize therapeutic outcomes.
