Author: Tiffany
Researchers have developed a novel nanoplatform utilizing small extracellular vesicles to deliver and visualize microRNA therapy in gastric cancer, potentially transforming treatment outcomes.
Key Highlights
- Research Question:
Can a nano-scale fluorescent complex encapsulated by small extracellular vesicles effectively deliver and visualize microRNA therapy in gastric cancer? - Research Difficulties:
Imaging the hybridization between microRNA and its target mRNA in vivo to assess therapeutic effects has been challenging. - Key Findings:
The new system significantly improved the efficiency of microRNA delivery, leading to enhanced cancer cell apoptosis in both in vitro and in vivo models. - Innovative Aspects:
This study introduces a real-time visualization method for gene therapy effectiveness via a fluorescent “off–on” switch using graphene quantum dots and microRNA. - Importance of the Study:
This research presents a refined theranostic platform that could improve the therapeutic efficacy of microRNA in combating gastric cancer, a malignancy with high mortality rates.
Gastric Cancer Treatment Challenges and miRNA Potential
Gastric cancer (GC) remains one of the leading causes of cancer-related mortality worldwide, with an alarming incidence rate particularly in East Asia. The disease often presents at advanced stages due to vague early symptoms, which include persistent abdominal discomfort and unexplained weight loss. Current treatment modalities, such as chemotherapy and targeted therapies, frequently suffer from limitations like drug resistance and systemic toxicity, reducing their overall effectiveness. The role of microRNAs (miRNAs) has garnered attention in cancer therapy, given their ability to regulate gene expression. However, effective delivery methods for miRNA therapeutics remain a significant challenge. This has propelled research into utilizing small extracellular vesicles (sEVs) as innovative delivery systems, given their biocompatibility and natural targeting capabilities. The development of a novel nanoplatform for miRNA delivery could enhance treatment outcomes for gastric cancer patients and address the pressing need for more effective therapeutic strategies.
Innovative Nanoplatform for miRNA Delivery
The research was conducted by a team led by Peiwen Fu and includes authors Yumeng Guo, Yanan Luo, Michael Mak, Jianguo Zhang, Wenrong Xu, Hui Qian, and Zhimin Tao, affiliated with Jiangsu University. Their findings were published in the Journal of Nanobiotechnology.
The primary aim of this research is to develop an effective and innovative delivery system for microRNA (miRNA) therapies that enables real-time visualization of their therapeutic effects in gastric cancer. Specifically, the study seeks to create a nanoplatform utilizing small extracellular vesicles (sEVs) to encapsulate and deliver miR-193a-3p, a known tumor suppressor, to gastric cancer cells. The objectives of the research include:
- Design a fluorescent complex of graphene quantum dots (GQDs) conjugated with miR-193a-3p for encapsulation in small extracellular vesicles (sEVs).
- Evaluate the efficiency of GQDs/miRNA@sEVs in delivering miR-193a-3p to gastric cancer cells, focusing on cellular uptake and endosomal escape.
- Establish a real-time imaging method to monitor miRNA-mRNA hybridization in vivo.
- Investigate the impact of delivered miR-193a-3p on target oncogenes, particularly cyclin D1, and assess resulting effects on cancer cell apoptosis.
- Contribute insights into the potential of sEVs as a versatile platform for miRNA delivery in cancer treatment.
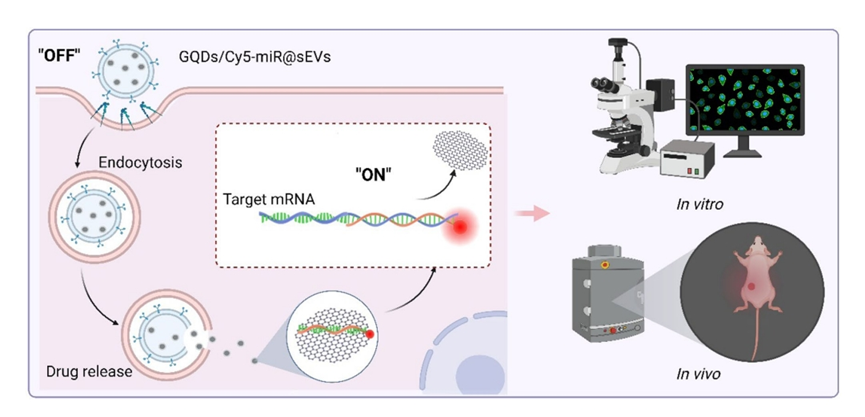
Figure 1. Graphical Abstract
Experimental Evaluation of GQDs/Cy5-miR@sEVs
Experimental Process Outline
- Isolation and culture of human umbilical cord mesenchymal stem cells (hucMSCs).
- Isolation and characterization of small extracellular vesicles (sEVs) from hucMSCs.
- Synthesis of graphene quantum dots (GQDs) and conjugation with miR-193a-3p to form GQDs/Cy5-miR complexes.
- Encapsulation of GQDs/Cy5-miR complexes into sEVs using tunable sonication.
- Evaluation of the stability of GQDs/Cy5-miR@sEVs using agarose gel electrophoresis and fluorescence measurements.
- In vitro assessment of cellular uptake and gene regulation in gastric cancer cells (HGC-27) treated with GQDs/Cy5-miR@sEVs.
- In vivo evaluation of therapeutic effects using a murine xenograft model of gastric cancer.
- Real-time imaging to monitor the biodistribution and therapeutic efficacy of GQDs/Cy5-miR@sEVs.
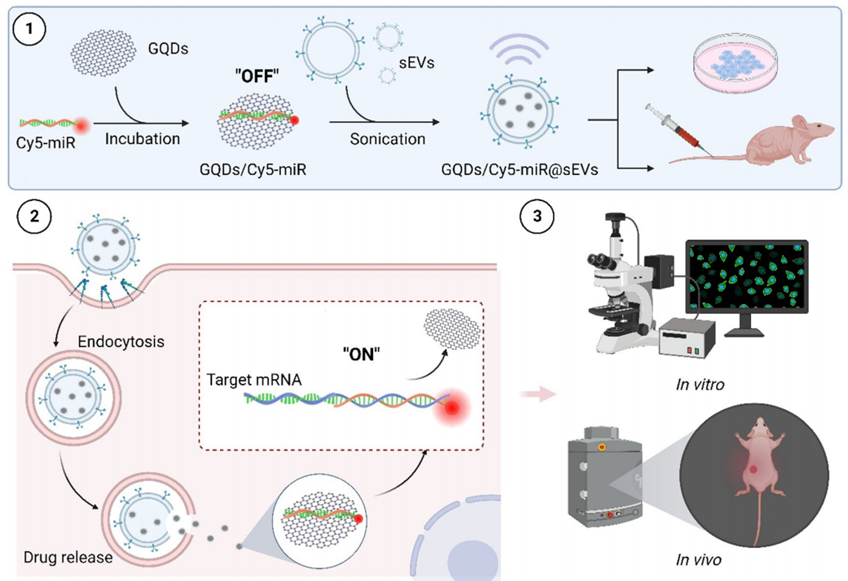
Figure 2. Illustrations that delineate the construct of GQDs/Cy5-miR@sEVs and their applications in the biological systems.
Key Experiments
1. Synthesis and Characterization of GQDs/Cy5-miR Complexes
Experimental Procedure:
GQDs were mixed with Cy5-tagged miR-193a-3p in a ratio of 30:1 in RNase-free Tris-HCl for 30 minutes at 37°C. The fluorescence quenching of Cy5 was measured in the presence of varying concentrations of GQDs.
Result:
The maximum quenching of Cy5 fluorescence was observed at (85.0 ± 0.4)% when optimal GQDs concentration was reached.
New Finding:
This experiment established that GQDs effectively quench Cy5 fluorescence, indicating successful conjugation and potential for fluorescent signal recovery upon target binding.
2. Stability Assessment of GQDs/Cy5-miR@sEVs
Experimental Procedure:
GQDs/Cy5-miR@sEVs were incubated in RNase solutions and subjected to agarose gel electrophoresis to evaluate the stability of the miRNA within the vesicles.
Result:
Agarose gel electrophoresis demonstrated that miRNA contents in the GQDs/Cy5-miR and GQDs/Cy5-miR complexes were degraded by RNases, while GQDs/Cy5-miR@sEVs showed a distinct band, indicating protection from degradation.
New Finding:
The encapsulation of GQDs/Cy5-miR in sEVs significantly enhances the stability of miRNA against RNase degradation, demonstrating the protective capability of the sEVs.
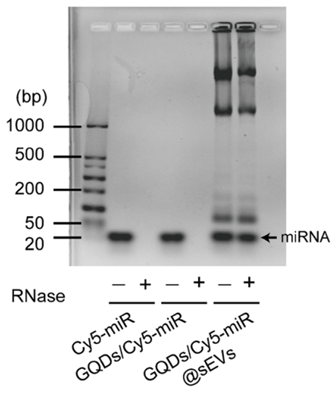
Figure 3. The degradation of Cy5-miR, GQDs/Cy5-miR and GQDs/Cy5-miR@sEVs in RNase solutions was detected by agarose gel electrophoresis.
3. In Vitro Assessment of Cellular Uptake
Experimental Procedure:
HGC-27 cells were treated with GQDs/FAM-miR@sEVs, and fluorescence imaging was performed at various time points (1, 2, and 4 hours) to assess cellular uptake and endosomal escape.
Result:
Confocal laser scanning microscopy showed high levels of FAM-miR uptake in HGC-27 cells, with FAM fluorescence detected in the cytoplasm and nucleus at 2 hours, continuing to glow at 4 hours.
New Finding:
The results indicate that GQDs/FAM-miR@sEVs facilitate effective cellular uptake and endosomal escape, allowing the miRNA to reach its target sites within the cells.
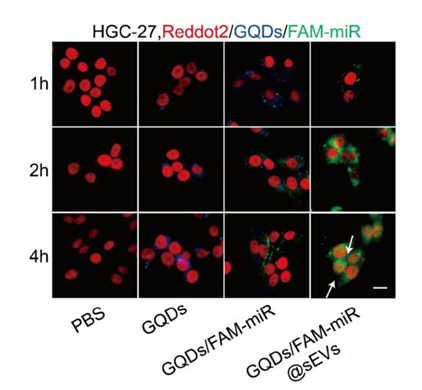
Figure 4. The fuorescence images showing the uptake of FAM-miR-GQDs@sEVs by HGC-27 cells at 1, 2, and 4 h. GQDs (blue), FAM-tagged miRNA (green) and nuclei (red) were shown, where scale bar =100 μm.
4. In Vivo Evaluation of Therapeutic Effects
Experimental Procedure:
BALB/c nude mice bearing HGC-27 tumors were injected with GQDs/Cy5-miR@sEVs, and real-time imaging was performed to monitor tumor accumulation and therapeutic effects over 24 hours.
Result:
GQDs/Cy5-miR@sEVs exhibited significantly higher fluorescence signals in tumors compared to Cy5-miR or GQDs/Cy5-miR, with peak accumulation at 1 hour post-injection.
New Finding:
This experiment revealed that GQDs/Cy5-miR@sEVs have superior tumor-targeting capability and can visualize the therapeutic effects of miR-193a-3p in a living organism, suggesting a promising approach for gastric cancer treatment.
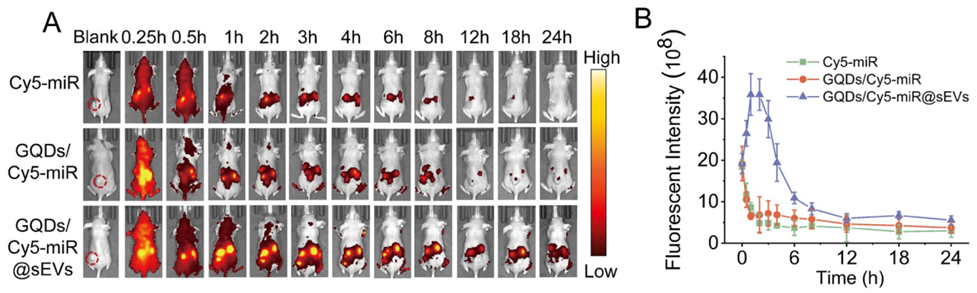
Figure 5. In vivo fluorescent “off–on” switch effect of GQDs/Cy5-miR@sEVs. (A) The fluorescent images of tumor-bearing mice after i.v. injection of GQDs/Cy5-miR@sEVs at time points as indicated. (B) The luorescent intensity of tumor sites in vivo at indicated time points.
Key Findings: Stability, Targeting, and Efficacy
This study presents significant advancements in the delivery and visualization of microRNA therapy for gastric cancer through the development of a novel nanoplatform composed of graphene quantum dots (GQDs) encapsulated within small extracellular vesicles (sEVs). Key findings include the successful synthesis of GQDs/Cy5-miR complexes, which demonstrated effective fluorescence quenching and recovery mechanisms, indicating successful target engagement. The encapsulation of these complexes in sEVs provided enhanced stability against RNase degradation and facilitated significant cellular uptake and endosomal escape of miR-193a-3p in gastric cancer cells. Additionally, in vivo experiments revealed that GQDs/Cy5-miR@sEVs exhibited superior tumor-targeting capabilities, leading to increased therapeutic effects and visualization of miRNA activity in a live murine model.
The innovation of this research lies in its dual functionality, combining therapeutic delivery with real-time imaging capabilities, thereby overcoming traditional limitations associated with miRNA therapies. This refined theranostic platform has the potential to transform treatment strategies for gastric cancer, a malignancy notorious for its poor prognosis and high mortality rates.
In the conclusion, the researchers state that the developed GQDs/Cy5-miRNA@sEVs nanoparticles serve as a powerful tool for visualizing the therapeutic efficacy of miRNA in both in vitro and in vivo settings. The study highlights the ability of this platform to provide real-time assessment of miRNA delivery and its biological effects, offering insights into the intricate dynamics of miRNA-mRNA interactions within cancer cells.
Reference:
Fu, Peiwen, et al. “Visualization of microRNA therapy in cancers delivered by small extracellular vesicles.” Journal of Nanobiotechnology 21.1 (2023): 457.
