Author: Tiffany
Researchers have developed a new formulation using nanospanlastics that improves the oral bioavailability and therapeutic efficacy of resveratrol in a mouse model of lipopolysaccharide-induced endotoxicity, indicating a significant advancement in drug delivery systems.
Key Highlights
- Research Question:
How can the bioavailability and therapeutic activity of resveratrol be improved for oral delivery in cases of endotoxicity? - Research Difficulties:
Resveratrol has poor bioavailability due to rapid metabolism and low solubility, limiting its therapeutic potential. - Key Findings:
The study demonstrated that RSV-loaded nanospanlastics outperformed conventional niosomes in reducing liver and kidney injury markers in mice. - Innovative Aspects:
The use of nanospanlastics as a flexible and biodegradable delivery system enhances the solubility and release profile of resveratrol, addressing previous limitations of traditional methods. - Importance of the Study:
This research offers a novel approach to enhance the efficacy of resveratrol, potentially improving treatment outcomes for conditions associated with systemic inflammation.
Context of Lipopolysaccharide-Induced Endotoxicity and Resveratrol’s Role
- Medical Condition: Lipopolysaccharide (LPS)-induced endotoxicity is a serious inflammatory response that can occur as a result of infections caused by gram-negative bacteria. This condition triggers systemic inflammation, which can escalate to septic shock and multiple organ failure, posing significant health risks if not managed promptly.
- Symptoms: Common symptoms include fever, tachycardia, hypotension, and altered organ function, which can lead to severe complications and increased mortality rates.
- Current Therapeutic Options: Resveratrol (RSV) has demonstrated potential anti-inflammatory effects; however, its clinical application is hampered by poor oral bioavailability and rapid metabolism. Traditional drug formulations often fail to deliver sufficient levels of RSV to achieve therapeutic effects, underscoring the need for innovative drug delivery systems that can enhance its efficacy in treating inflammatory conditions.
Development Goals for Enhanced Resveratrol Delivery
The research team, comprising Mostafa Mohamed Younis, Noha Abd El-Fattah Fadel, Asmaa Badawy Darwish, and Amira Mohamed Mohsen from the National Research Centre and Egyptian Atomic Energy Authority, published their findings in the Journal of Pharmaceutical Innovation on March 4, 2023.
The primary aim of this research is to develop a novel formulation of RSV-loaded nanospanlastics to enhance the oral delivery and therapeutic activity of resveratrol in a model of LPS-induced endotoxicity in mice. This study seeks to address the challenges associated with RSV’s poor bioavailability and rapid metabolism, which limit its clinical use. By employing nanospanlastics, the research intends to improve the solubility and sustained release of resveratrol, thereby increasing its concentration at the target sites. Additionally, the study aims to evaluate the effectiveness of this formulation in mitigating organ damage and inflammatory responses in the mouse model, ultimately contributing to the development of more effective treatments for systemic inflammatory conditions.
Formulation and Evaluation of RSV-Loaded Nanospanlastics
1. Experimental Process:
- Development of RSV-loaded nanospanlastics using the thin film hydration technique.
- Characterization of formulations, including measurements of vesicular size (VS), polydispersity index (PDI), and zeta potential (ZP).
- Conducting Fourier Transform Infrared (FT-IR) spectroscopy analysis to assess interactions between RSV and components.
- Performing Transmission Electron Microscopy (TEM) to evaluate the morphology of formulations.
- Conducting in vitro release studies using the dialysis bag diffusion technique.
- Implementing in vivo efficacy testing using a lipopolysaccharide (LPS)-induced endotoxicity model in mice
2. Key Experiments:
(1) Preparation of RSV-Loaded Nanospanlastics
Procedure: The formulations were developed using the thin film hydration technique, incorporating nonionic surfactants and edge activators. The drug-loaded nanospanlastics were characterized for entrapment efficiency (EE%), vesicular size (VS), and zeta potential (ZP).
Result: The entrapment efficiencies ranged from 45.84 ± 0.039% to 85.03 ± 0.022%. The particle sizes varied between 260.5 ± 52.17 nm and 794.3 ± 97.78 nm, with zeta potential values indicating good stability (≤ −20 mV).
New Findings: These results demonstrate the successful formulation of RSV-loaded nanospanlastics with high entrapment efficiency and suitable particle size, which are essential for effective drug delivery.
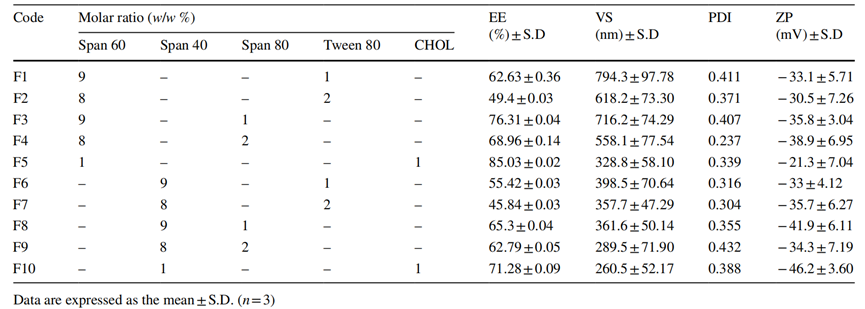
Table 1. Composition, entrapment efciency and physicochemical properties of RSV-loaded nanospanlastics/niosomes.
(2) In Vitro Release Studies
Procedure: The in vitro release profile was assessed using the dialysis bag diffusion technique, with samples taken at various time intervals over a 24-hour period.
Result: The cumulative release of RSV from formulations F3 and F8 after 8 hours was 47.32 ± 2.87% and 63.48 ± 6.05%, respectively. The release exhibited a biphasic pattern, transitioning from rapid to sustained release.
New Findings: The release mechanism was determined to be Fickian diffusion-controlled, with n values of 0.1734 for F3 and 0.1959 for F8, indicating effective drug release characteristics.
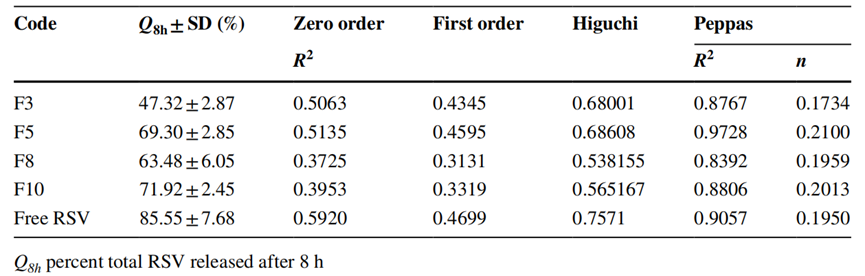
Table 2. The calculated correlation coefcients and kinetics parameters of RSV release profle from diferent formulations.
(3) In Vivo Efficacy Testing
Procedure: The efficacy of RSV-loaded nanospanlastics (F8) was evaluated in a mouse model of LPS-induced endotoxicity. Mice were treated with F8, and serum levels of liver and kidney function markers were measured post-treatment.
Result: Treatment with F8 resulted in a significant reduction in serum levels of ALT by 22%, AST by 18%, and creatinine by 24% compared to the LPS group.
New Findings: These findings highlight the potential of RSV-loaded nanospanlastics in mitigating organ damage and inflammation, demonstrating enhanced therapeutic efficacy compared to free RSV and conventional niosomes.
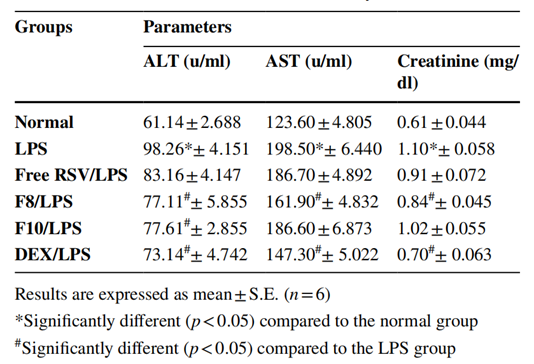
Table 3. Efect of RSV formulations on the serum levels of ALT, AST, and creatinine, in LPS-induced endotoxicity in mice.
Key Findings and Implications
This study successfully demonstrated that RSV-loaded nanospanlastics significantly enhance the oral delivery and therapeutic efficacy of resveratrol in a mouse model of LPS-induced endotoxicity. The key findings revealed high entrapment efficiencies, optimal particle sizes, and a favorable in vitro release profile for the developed formulations. Notably, in vivo results indicated that treatment with RSV-loaded nanospanlastics led to substantial reductions in serum levels of liver and kidney function markers, emphasizing their ability to mitigate organ damage and inflammation more effectively than conventional formulations. This research underscores the innovation of utilizing nanospanlastics as a flexible and biodegradable drug delivery system, which holds promise for improving the clinical application of resveratrol in treating systemic inflammatory conditions.
Reference:
Younis, Mostafa Mohamed, et al. “Nanospanlastics as a novel approach for improving the oral delivery of resveratrol in lipopolysaccharide-induced endotoxicity in mice.” Journal of Pharmaceutical Innovation 18.3 (2023): 1264-1278.
