Editor: Nina
This study develops hyaluronic acid-coated thiolated chitosan nanoparticles for the targeted delivery and sustained release of Cisplatin in cervical cancer, enhancing drug efficacy and minimizing off-target toxicity through selective CD44 receptor targeting.
Key Preview
Research Question:
The study seeks to minimize the side effects of Cisplatin in cervical cancer treatment by using nanoparticles to specifically target CD44 overexpressing cancer cells, enhancing drug delivery precision.
Research Design and Strategy:
The research employs a green synthesis approach to prepare hyaluronic acid-coated thiolated chitosan nanoparticles, enabling the targeted delivery of Cisplatin to cancer cells expressing CD44.
Method:
The ionic gelation method was used to create nanoparticles by crosslinking thiolated chitosan with tripolyphosphate (TPP), followed by Cisplatin encapsulation, and the nanoparticles were characterized for size, surface charge, and drug encapsulation efficiency.
Key Results:
The nanoparticles had an average size of 265.9 nm, a zeta potential of +22.3 mV, a drug loading efficiency of 70.1%, and an entrapment efficiency of 45%, with drug release following the Higuchi model for up to 72 hours; in vitro studies revealed increased cytotoxicity in HeLa cells and reduced toxicity in HCK1T cells.
Significance of the Research:
This study demonstrates a novel approach for improving Cisplatin delivery to cervical cancer cells, reducing off-target toxicity, and providing sustained drug release, offering a more effective alternative to traditional chemotherapy.
Introduction
Nanoparticle-based drug delivery systems have revolutionized the way chemotherapeutic agents are delivered to cancer cells, allowing for more effective and less toxic treatments. In this study, the authors introduced a novel drug delivery system using hyaluronic acid-coated thiolated chitosan nanoparticles to target CD44 receptors, which are overexpressed in cervical cancer cells. This method not only ensures the precise delivery of Cisplatin but also reduces its harmful side effects, a significant concern in traditional chemotherapy.
Research Team and Objective
The research was conducted by a team of experts from various institutions, including the National University of Sciences and Technology, Pakistan, and King Abdulaziz University, Saudi Arabia. The study, titled “Green synthesis of hyaluronic acid-coated thiolated chitosan nanoparticles for CD44 targeted delivery and sustained release of Cisplatin in cervical carcinoma,” was published in Frontiers in Pharmacology in January 2023. The objective was to develop a biocompatible, non-toxic, and effective drug delivery system to reduce the adverse effects of Cisplatin and improve its anticancer efficacy.
Experimental Process
Outline:
1. Preparation of Thiolated Chitosan (ThCs)
2. Nanoparticle Formation Using Ionic Gelation
3. Coating the Nanoparticles with Hyaluronic Acid (HA)
4. Drug Loading and Encapsulation Efficiency
5. In Vitro Drug Release Studies
6. Cytotoxicity Assays (MTT and Trypan Blue Exclusion)
1. Preparation of Thiolated Chitosan (ThCs)
Key Steps:
- Chitosan Solution Preparation: Chitosan was dissolved in 1% acetic acid to form a 1% chitosan solution.
- Thiolation Reaction: Thioglycolic acid (TGA) was added to the chitosan solution at a concentration of 6.9 mL of TGA per 1% chitosan solution. To initiate the reaction, 50 mM of EDC (a crosslinker) was added, activating the carboxyl groups of TGA to form amide bonds with the amino groups of chitosan.
- Dialysis and Lyophilization: The mixture was dialyzed for 72 hours to remove any unreacted TGA, followed by lyophilization to obtain the final thiolated chitosan.
Results and Key Data:
- The resulting thiolated chitosan was a white, fibrous, and odorless material.
- The thiol group content was quantified using Ellman’s reagent, which showed a thiol substitution of 787 μmol/G.
Significance of the Result:
- Thiolated chitosan is an essential building block for the nanoparticle formulation. The high thiol group content enables the nanoparticles to interact effectively with cancer cells and provides the basis for the subsequent encapsulation of Cisplatin. This step ensures that the nanoparticles have the required functionality to target and deliver drugs effectively to the tumor site.
Key Innovations:
- The use of thiolated chitosan allows for the formation of nanoparticles that can be easily modified for drug delivery. The thiolation process enhances the biocompatibility and mucoadhesive properties of chitosan, which are essential for efficient drug targeting and retention in the tumor microenvironment.
2. Nanoparticle Formation Using Ionic Gelation
Key Steps:
- Solution Preparation: Thiolated chitosan (ThCs) was dissolved in distilled water to form a homogeneous solution.
- Ionic Gelation: Tripolyphosphate (TPP), an anionic crosslinker, was added dropwise to the ThCs solution under constant stirring, promoting ionic gelation and nanoparticle formation.
- Sonication: The nanoparticle dispersion was sonicated for 15 minutes to ensure uniform dispersion and to minimize agglomeration.
Results and Key Data:
- The resulting nanoparticles had an average size of 265.9 nm, a zeta potential of +22.3 mV, and a polydispersity index (PDI) of 0.226, indicating a narrow size distribution and good stability.
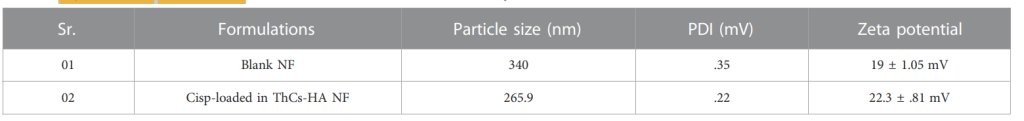
Table 1. Physiochemical characteristics of blank ThCs and Cis loaded ThCs nanoparticles.
- The size and charge of the nanoparticles were optimal for efficient cellular uptake and stability in biological systems.
Significance of the Result:
- The size of the nanoparticles (265.9 nm) is ideal for passive accumulation in tumor tissue via the enhanced permeation and retention (EPR) effect. The positive zeta potential enhances the interaction between the nanoparticles and the negatively charged cancer cell membranes, promoting targeted drug delivery.
- The PDI value indicates that the nanoparticle formulation is homogeneous, which ensures consistent drug delivery.
Key Innovations:
- The ionic gelation method used in this study is simple and avoids the use of harsh chemicals, making it a green, environmentally friendly approach to nanoparticle synthesis. This method also allows for easy scaling up, making it suitable for clinical applications.
3. Coating the Nanoparticles with Hyaluronic Acid (HA)
Key Steps:
- HA Solution Preparation: Hyaluronic acid (HA) was dissolved in distilled water to form a solution.
- Surface Coating: The HA solution was added to the ThCs nanoparticle dispersion. The carboxyl groups of HA interacted with the amine groups of ThCs, resulting in the formation of HA-coated nanoparticles.
Results and Key Data:
- The zeta potential of the nanoparticles increased from +19 mV (for uncoated nanoparticles) to +22.3 mV after the addition of HA, confirming successful coating of the nanoparticles.
Significance of the Result:
- Coating the nanoparticles with HA functionalizes their surface, enabling selective targeting of CD44 receptors, which are overexpressed on cervical cancer cells. This modification enhances the specificity of drug delivery, reducing off-target effects and improving the therapeutic index of Cisplatin.
- The positive charge on the HA-coated nanoparticles facilitates their interaction with cancer cell membranes, improving cellular uptake.
Key Innovations:
- The use of hyaluronic acid for surface modification is an innovative strategy to target specific receptors on cancer cells. HA’s natural biocompatibility and affinity for CD44 receptors provide a unique and effective means of targeting cancer cells while minimizing damage to healthy cells.
4. Drug Loading and Encapsulation Efficiency
Key Steps:
- Drug Loading: Cisplatin was dissolved in a suitable solvent and added to the HA-ThCs nanoparticle dispersion. The mixture was stirred and sonicated for 15 minutes to ensure uniform drug incorporation into the nanoparticles.
- Nanoparticle Collection: The drug-loaded nanoparticles were centrifuged to remove any unencapsulated Cisplatin, and the supernatant was analyzed to determine the amount of free drug.
- Lyophilization and Storage: The drug-loaded nanoparticles were lyophilized and stored for further use.
Results and Key Data:
- The drug loading efficiency (DL) was found to be 70.1%, and the encapsulation efficiency (EE) was 45%, indicating effective incorporation of Cisplatin into the nanoparticles.
- The successful encapsulation of Cisplatin ensures a sufficient drug dose is available for targeted release at the tumor site.
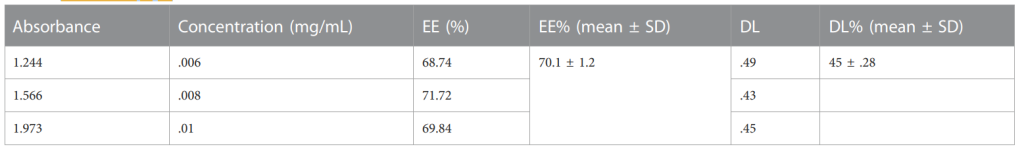
Table 2.Percentages of EE and DL of NFs of Cis loaded in HA-ThCs (p < .05 mean ± S.D).
Significance of the Result:
- High drug loading and encapsulation efficiencies are crucial for achieving therapeutic doses while minimizing drug loss. The efficiency of drug encapsulation also indicates the stability of the nanoparticle formulation, ensuring that Cisplatin remains intact and bioavailable for extended release.
Key Innovations:
- The combination of hyaluronic acid and thiolated chitosan allows for high drug encapsulation, providing a platform for efficient drug delivery. The encapsulation of Cisplatin within nanoparticles also offers the potential for sustained release, minimizing the need for frequent dosing.
5. In Vitro Drug Release Studies
Key Steps:
- Release Medium Preparation: Phosphate-buffered saline (PBS) at pH 6.8 (representing the acidic tumor microenvironment) and pH 7.4 (representing normal physiological conditions) were used to simulate the release conditions.
- Release Study: The Cisplatin-loaded nanoparticles were placed in a dialysis bag, and the release was monitored over a 72-hour period. Samples were taken at various time intervals and analyzed for the amount of Cisplatin released using UV-Vis spectroscopy at 269 nm.
Results and Key Data:
- The drug release profile showed sustained release over 72 hours, with 66.8% of Cisplatin released at pH 6.8 and 80.5% released at pH 7.4.
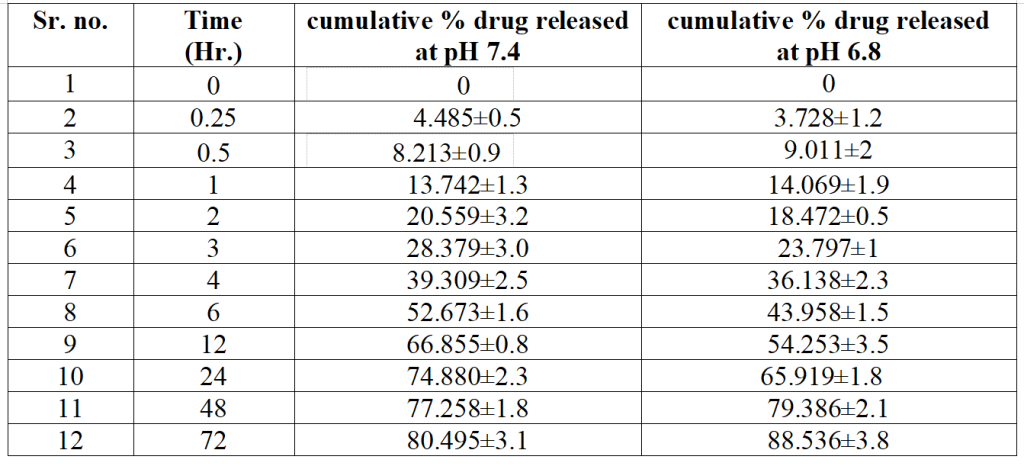
Table 3. Percentage of Cis released in phosphate buffer at pH 7.4 and 6.8 at specific time intervals for determination of best kinetic model
- The release followed the Higuchi model, indicating drug release via diffusion rather than erosion or dissolution of the nanoparticle matrix.
Significance of the Result:
- The sustained release of Cisplatin over 72 hours provides prolonged therapeutic efficacy, reducing the need for frequent drug administration. This is particularly advantageous for chemotherapy, as it minimizes side effects and improves patient compliance.
- The pH-sensitive release mechanism ensures that the drug is primarily released in the acidic tumor microenvironment, enhancing targeted drug delivery to cancer cells while minimizing release in normal tissues.
Key Innovations:
- The sustained and pH-sensitive release of Cisplatin is a key innovation of this nanoparticle system. The use of the Higuchi diffusion model to describe drug release allows for better control over the release kinetics, ensuring that Cisplatin is released at a steady rate over time.
6. Cytotoxicity Assays (MTT and Trypan Blue Exclusion)
Key Steps:
- Cell Culture: HeLa cervical cancer cells and HCK1T normal cervical epithelial cells were cultured under standard conditions.
- Treatment with Nanoparticles: Cells were treated with various concentrations of Cisplatin and HA-ThCs-Cis nanoparticles for 24 hours.
- MTT Assay: The MTT assay was performed to measure cell metabolic activity as an indicator of cell viability.
- Trypan Blue Exclusion: The trypan blue exclusion assay was used to determine cell membrane integrity and apoptosis.
Results and Key Data:
- HeLa cells treated with HA-ThCs-Cis nanoparticles exhibited significantly lower viability (IC50 of 22 μg/mL after 24 hours) compared to those treated with free Cisplatin (IC50 of 26 μg/mL).
- HCK1T cells showed higher viability when treated with HA-ThCs-Cis nanoparticles, indicating selective toxicity toward cancer cells.
Significance of the Result:
- The selective toxicity of the HA-ThCs-Cis nanoparticles toward cancer cells, with minimal effects on normal cells, is a major advantage for targeted chemotherapy. This result confirms the ability of the HA-coated nanoparticles to deliver Cisplatin more specifically to cancer cells, reducing off-target toxicity.
Key Innovations:
- The use of HA for targeting CD44 receptors on cancer cells results in enhanced drug uptake by cancer cells and reduced uptake by normal cells, showcasing the nanoparticle’s potential for selective drug delivery and minimal side effects.
Conclusion
The study demonstrated that hyaluronic acid-coated thiolated chitosan nanoparticles could effectively deliver Cisplatin to cervical cancer cells, with improved cytotoxicity and reduced off-target effects. The nanoparticles’ sustained drug release profile and enhanced cellular uptake make this formulation a promising candidate for targeted cancer therapy. Further research is needed to explore the long-term stability and in vivo efficacy of these nanoparticles, with potential applications in other types of cancer.
Reference:
Kousar, Kousain, et al. “Green Synthesis of Hyaluronic Acid-Coated, Thiolated Chitosan Nanoparticles for CD44 Targeted Delivery and Sustained Release of Cisplatin in Cervical Carcinoma.” Frontiers in Pharmacology, vol. 13, 2023, p. 1073004.
