Editor:Nina
This study demonstrates that incorporating Pam2Cys into mRNA-lipid nanoparticles significantly enhances immune responses, providing a potent and versatile platform for mRNA vaccines against cancer and infectious diseases.
Key Preview
Research Question
The study examines how incorporating Pam2Cys into mRNA vaccines enhances their immunological efficacy, addressing the limitation of suboptimal immune responses in existing formulations.
Research Design and Strategy
The researchers implemented experiments using both prophylactic and therapeutic models to assess the effects of Pam2Cys in mRNA-LNPs, focusing on tumor suppression and infection prevention, including a COVID-19 surrogate model.
Method
Pam2Cys was co-delivered with mRNA in lipid nanoparticles, and immune responses were analyzed through cytokine profiling, antigen presentation, and evaluations of tumor inhibition and antibody responses in murine models.
Key Results
Pam2Cys-mRNA vaccines significantly boosted pro-inflammatory cytokines, improved tumor suppression in 50% of treated mice, and exhibited strong humoral responses, including robust performance in a COVID-19 model.
Significance of the Research
The findings highlight Pam2Cys as a potent adjuvant for mRNA vaccines, advancing their application against various diseases and offering a promising pathway for clinical improvements.
Introduction
The evolution of mRNA vaccines has accelerated remarkably in recent years, especially during the COVID-19 pandemic, showcasing their potential as both prophylactic and therapeutic agents. This rapid development has prompted extensive research focused on enhancing the efficacy of mRNA vaccines. Despite their promise, existing formulations often struggle to elicit robust immune responses, a challenge that necessitates ongoing innovation. The current study introduces Pam2Cys, a Toll-like receptor 2/6 agonist, as a novel adjuvant to improve mRNA vaccine performance. The primary objective is to determine whether Pam2Cys can significantly augment the immune response generated by mRNA vaccines, ultimately addressing the limitations of current formulations.
Research Team and Objective
The study was conducted by a team of researchers consisting of Yangzhuo Gu, Jingyun Yang, Cai He, Tingmei Zhao, Ran Lu, Jian Liu, Xianming Mo, Fuqiang Wen, and Huashan Shi, all affiliated with the State Key Laboratory of Biotherapy and Cancer Center at West China Hospital, Sichuan University. The paper, titled “Incorporation of a Toll-like receptor 2/6 agonist potentiates mRNA vaccines against cancer and infectious diseases,” was published in the journal Signal Transduction and Targeted Therapy. The research aims to significantly enhance the efficacy and safety of mRNA vaccines through the strategic integration of Pam2Cys, thereby paving the way for improved vaccine strategies.
Experimental Process
Formulation and Characterization of Pam2Cys-Incorporated mRNA-LNPs
Key Steps:
Researchers formulated mRNA-LNPs by incorporating Pam2Cys into a lipid mixture containing ionizable cationic lipids, DSPC, cholesterol, and PEGylated lipids. Using a microfluidic mixing device, the mRNA and lipid solutions were combined to form nanoparticles. The formulations were optimized with a 0.5% molar ratio of Pam2Cys, ensuring the integration of this adjuvant into the lipid bilayer. Key parameters, including particle size, polydispersity index (PDI), zeta potential, and encapsulation efficiency, were assessed using dynamic light scattering and fluorescence assays.
Results and Key Data:
The Pam2Cys-mRNA-LNPs exhibited an average size of 82.7 ± 2.4 nm, a PDI of 0.11 ± 0.01, and a zeta potential of −4.18 ± 0.83 mV, all comparable to conventional mRNA-LNPs. Encapsulation efficiency was also high (90.9 ± 0.7%), ensuring robust delivery potential.
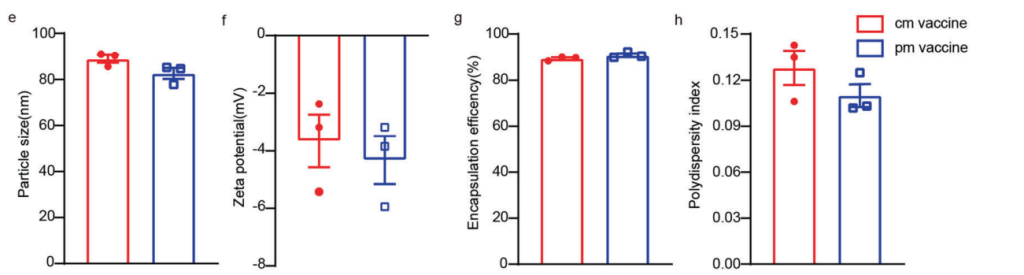
Figure 1. e–h Particle size, zeta potential, encapsulation efficiency and polydispersity index of OVA cm vaccine and OVA pm vaccine.
Significance of the Results:
These findings confirm that the inclusion of Pam2Cys does not compromise the physical properties of mRNA-LNPs, ensuring their stability and suitability for immunization. Maintaining encapsulation efficiency is critical for delivering sufficient mRNA payloads.
Key Innovations:
The use of Pam2Cys as a lipid-compatible adjuvant eliminates the need for chemical modifications or additional formulation steps, offering a scalable and efficient strategy to enhance mRNA vaccine efficacy.
Immune Profiling in Draining Lymph Nodes
Key Steps:
Mice were immunized intramuscularly with Pam2Cys-mRNA-LNPs. Twenty-four hours post-vaccination, draining lymph nodes (dLNs) were harvested for analysis. Cytokine concentrations were measured using multiplex assays, while dendritic cell subsets were analyzed by flow cytometry to evaluate antigen presentation and activation. The activation markers CD86 and MHC-I antigen presentation levels were assessed to determine dendritic cell functionality.
Results and Key Data:
The Pam2Cys-LNPs significantly increased levels of IL-12 (Th1 cytokine) and IL-17 (Th17 cytokine) compared to conventional mRNA-LNPs. Migratory and resident cDC2s demonstrated enhanced antigen presentation and upregulated CD86 expression, indicating effective activation.
Significance of the Results:
Elevated IL-12 and IL-17 levels signify a bias toward Th1 and Th17 responses, which are essential for tumor immunity and robust antigen-specific responses. Enhanced dendritic cell activation facilitates effective priming of T cells, a critical step in generating adaptive immunity.
Key Innovations:
This study demonstrates the ability of Pam2Cys to modulate the immune microenvironment in dLNs, tailoring cytokine profiles for optimal immune activation. This represents a strategic improvement in mRNA vaccine adjuvant design.
Therapeutic Tumor Model Studies
Key Steps:
Mice were subcutaneously inoculated with E.G7-OVA tumor cells and treated three days later with a single intramuscular dose of Pam2Cys-mRNA-LNPs encoding ovalbumin (OVA). Tumor growth was monitored over time, and T cell responses were assessed through flow cytometry. Survival rates and tumor regression rates were also recorded.
Results and Key Data:
The vaccine induced complete tumor regression in 50% of treated mice, whereas no tumor regression was observed in the control group. Flow cytometry revealed robust CD4+ and CD8+ T cell activation, both critical for tumor clearance. Survival rates in the Pam2Cys group were significantly higher compared to the control.
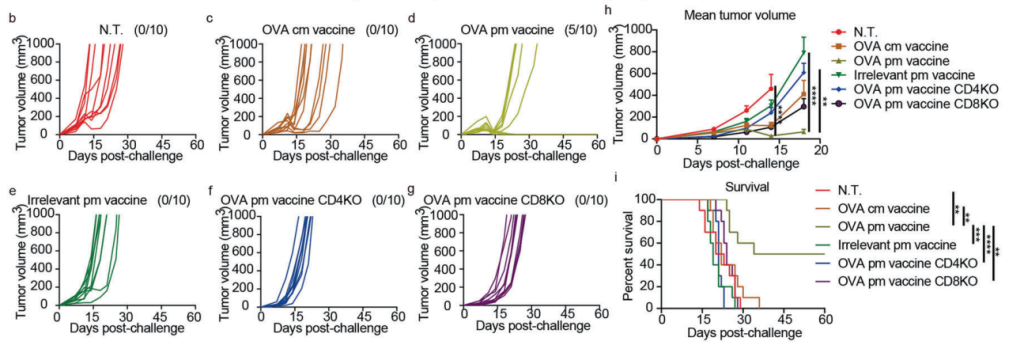
Figure 2. b–g Individual growth curves of E.G7-OVA tumors in mice in therapeutic assay. Fractions in the parentheses indicate the proportion of cured mice in the groups.
Significance of the Results:
These results highlight the vaccine’s potential as a therapeutic agent for established tumors. The induction of T cell-mediated immunity underscores its ability to provide durable and specific antitumor responses.
Key Innovations:
This approach successfully integrates Pam2Cys as an adjuvant to boost T cell activation and memory formation, demonstrating efficacy against solid tumors, a key challenge in cancer immunotherapy.
Evaluation in a Surrogate COVID-19 Model
Key Steps:
Mice were immunized with Pam2Cys-mRNA-LNPs encoding the SARS-CoV-2 spike protein. After 14 days, sera were collected to measure RBD-specific IgG and pseudovirus-neutralizing antibody titers. Splenocytes were harvested for intracellular cytokine staining to assess T cell activation.
Results and Key Data:
The vaccine elicited significantly higher RBD-binding IgG titers and neutralizing antibodies compared to conventional formulations. T cell analysis showed increased frequencies of IFN-γ-producing CD8+ and CD4+ T cells, indicating robust cellular immunity.
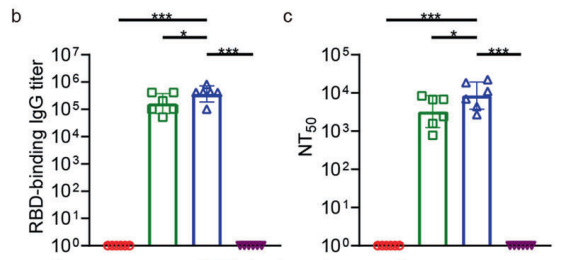
Figure 3. b Measurement of RBD-binding IgG titers in peripheral blood. c Measurement of neutralization antibody titers in peripheral blood against spike pseudoviruses. NT50, neutralizing antibodies with 50% neutralization titer. Data are shown as geometric means with SD.
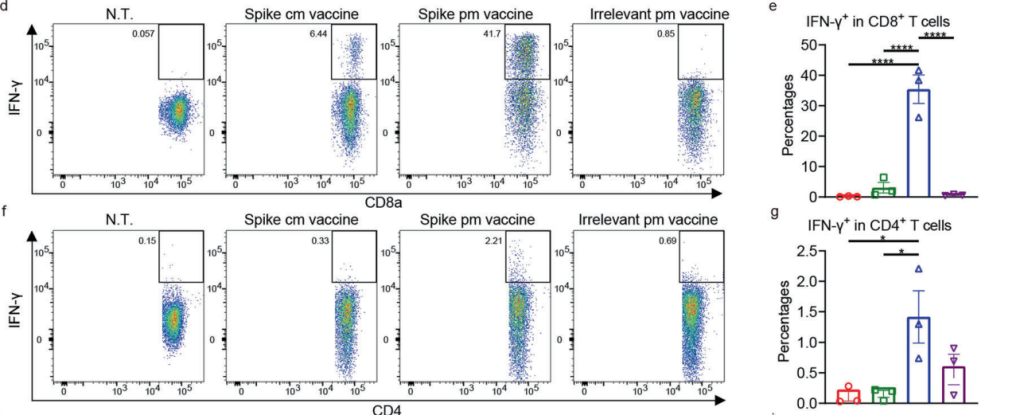
Figure 4. d, e Ratios of IFN-γ+ cells in CD8+ T cells. f, g Ratios of IFN-γ+ cells in CD4+ T cells.
Significance of the Results:
These findings confirm the vaccine’s ability to induce both humoral and cellular immune responses, critical for protection against SARS-CoV-2 and other infectious diseases.
Key Innovations:
The dual enhancement of antibody production and T cell activation demonstrates the versatility of Pam2Cys in addressing diverse immunological challenges.
Preliminary Safety Assessment
Key Steps:
Mice were monitored for weight changes, behavior, and signs of toxicity for 70 days post-immunization. Vital organs were collected and examined histologically for any pathological changes.
Results and Key Data:
No weight loss, behavioral abnormalities, or histopathological changes were observed in the Pam2Cys-mRNA-LNP group.
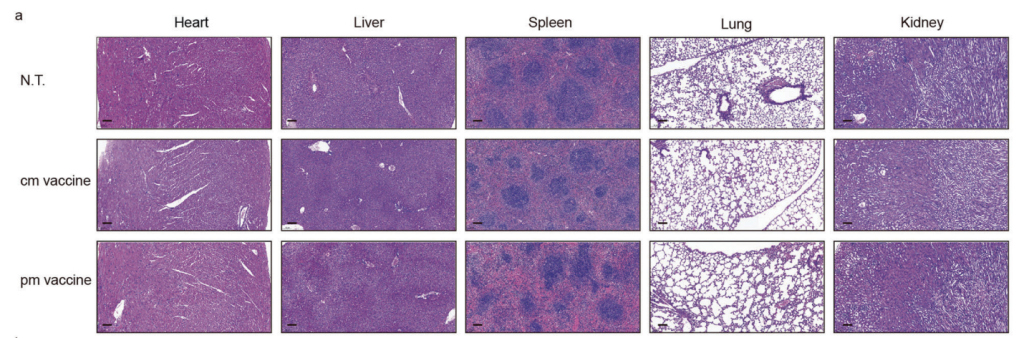
Figure 5. Histological sections of vital organs of immunized mice 45 days after the last vaccination. Scale bar on the lower left corner of each snapshot, 100 μm.
Significance of the Results:
The absence of adverse effects supports the safety of Pam2Cys incorporation, a critical step for potential clinical translation.
Key Innovations:
The compatibility of Pam2Cys with mRNA-LNP formulations ensures that safety is not compromised while enhancing efficacy, addressing a critical challenge in vaccine development.
Conclusion
The study presents compelling evidence that the incorporation of Pam2Cys into mRNA vaccines significantly enhances their efficacy against cancer and infectious diseases. The findings indicate that this innovative approach successfully stimulates both cellular and humoral immune responses, providing a strong foundation for future clinical applications. However, the research is not without limitations, including the need for further validation in human trials and a more in-depth understanding of the mechanisms at play. Future studies should explore the potential of Pam2Cys in various vaccine contexts, optimizing formulations to leverage its adjuvant properties for improved immunogenicity.
Reference:
Gu, Yangzhuo, et al. “Incorporation of a Toll-like receptor 2/6 agonist potentiates mRNA vaccines against cancer and infectious diseases.” Signal Transduction and Targeted Therapy 8.1 (2023): 273.
