Author: Tiffany
A recent study published in the International Journal of Molecular Sciences suggests that Asiaticoside (AS) could potentially reduce symptoms of Alzheimer’s disease (AD) by targeting inflammation and supporting synaptic repair in animal models, offering insights into alternative treatment avenues for AD.
Key Highlights:
- Research Question:
Can Asiaticoside mitigate Alzheimer’s disease symptoms by reducing neuroinflammation and promoting synaptic repair? - Research Difficulties:
A challenge was demonstrating AS’s efficacy in crossing the blood-brain barrier and targeting multiple AD-related mechanisms. - Key Findings:
AS improved cognitive function in AD mice by reducing neuroinflammation and enhancing synaptic repair. - Innovative Aspects:
This study is the first to show that AS targets both inflammation and synaptic dysfunction in AD. - Importance of the Study:
The findings suggest AS could offer a dual therapeutic approach to address key pathologies of Alzheimer’s disease.
Alzheimer’s Disease and Current Treatment Limitations
Overview of Alzheimer’s Disease
Alzheimer’s disease (AD) is a chronic neurodegenerative disorder, predominantly affecting older individuals, and it remains a leading cause of dementia. The disease is characterized by the accumulation of amyloid-beta (Aβ) plaques and tau protein tangles, which contribute to neuronal damage and significant cognitive decline, including deficits in memory, thinking, and reasoning.
Symptoms and Progression
The early stages of AD often present as mild memory loss and confusion, which gradually worsen. As the disease advances, cognitive impairments intensify, manifesting as disorientation, mood changes, and an inability to carry out everyday tasks. In its later stages, AD patients typically require continuous care as they lose their independence.
Current Treatment Approaches and Their Limitations
The available treatments for AD primarily focus on providing symptomatic relief, with drugs targeting neurotransmitter systems such as cholinergic and glutamatergic pathways. However, these treatments do not address the underlying causes of AD and are limited to slowing the disease’s progression rather than halting or reversing its course.
Research Objectives: Exploring Asiaticoside as a Potential Treatment for AD
Focus on Asiaticoside (AS)
The aim of the study was to assess the effects of Asiaticoside (AS), a compound derived from Centella Asiatica, in mitigating the symptoms of Alzheimer’s disease. Specifically, the researchers explored AS’s potential to reduce neuroinflammation and enhance synaptic function, both of which are crucial factors contributing to cognitive decline in AD.
Research Team and Publication
The study was carried out by Sai Liu and colleagues from China Pharmaceutical University and The Ohio State University, and was published in the International Journal of Molecular Sciences in July 2023. The findings add valuable knowledge regarding AS’s neuroprotective properties and its possible role in treating AD.
Experimental Process and Research Findings
Experimental Process Overview:
- Induction of Alzheimer’s in Mice:
- Administration of Asiaticoside:
- Cognitive Function Testing:
- Histological and Molecular Analysis:
- Gene Expression Profiling:
Key Experiments:
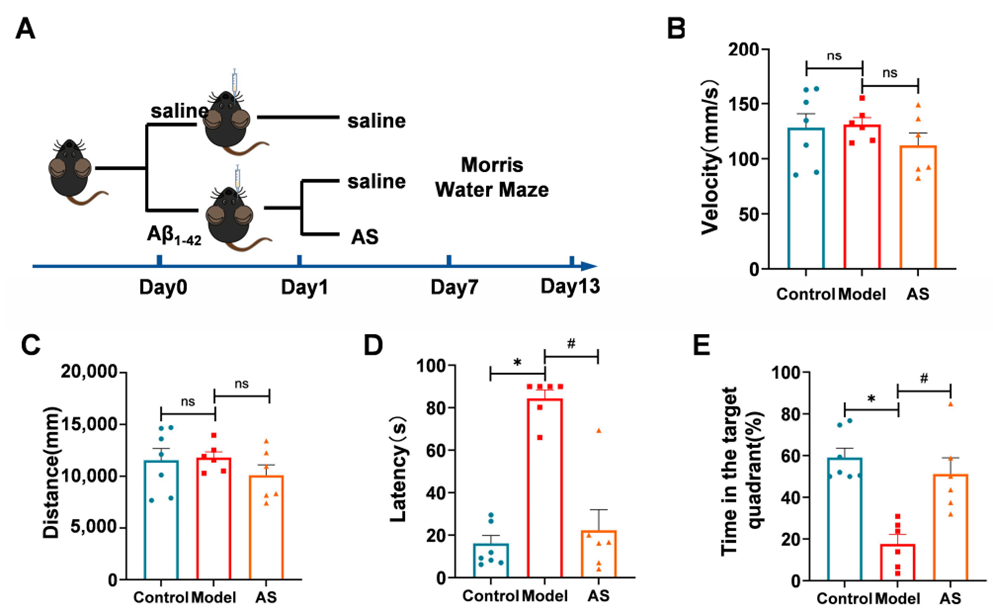
- Cognitive Function Assessment via Morris Water Maze:
- Preparatory Work:
Aβ1-42 was injected into the lateral ventricles of mice to induce AD-like pathology. AS treatment was given starting from day 1. - Experimental Procedure:
Mice underwent a 5-day training period followed by a probe trial on day 6 to assess spatial learning and memory. - Results:
- AS-treated mice exhibited significant improvements in cognitive function compared to the model group, showing a reduction in latency and increased time spent in the target quadrant.
- New Findings:
AS treatment led to a notable improvement in spatial learning and memory, demonstrating its potential to enhance cognitive function in AD models.
- Preparatory Work:
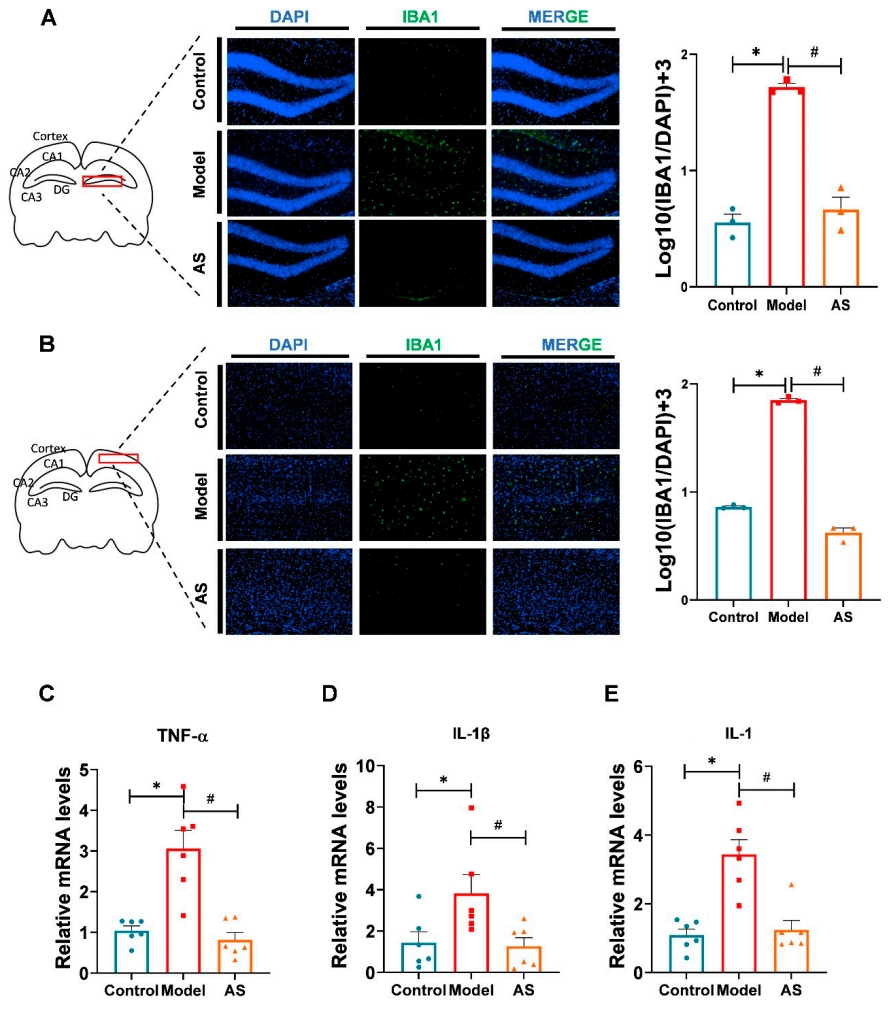
- Anti-inflammatory Effects of Asiaticoside:
- Preparatory Work:
Mice brains were dissected for immunofluorescence analysis to observe microglial activation and proinflammatory cytokine expression. - Experimental Procedure:
Immunofluorescence staining for IBA1 (microglial marker) was performed, and RT-PCR was used to assess mRNA levels of proinflammatory cytokines (TNF-α, IL-1β, IL-6). - Results:
- AS treatment inhibited microglial activation in both the cortex and hippocampus, as evidenced by reduced IBA1-positive cell counts.
- Proinflammatory cytokines (TNF-α, IL-1β, IL-6) were downregulated in AS-treated mice, demonstrating reduced neuroinflammation.
- New Findings:
AS effectively reduced neuroinflammation, suggesting its role in modulating microglial activation and suppressing the inflammatory cascade associated with AD.
- Preparatory Work:
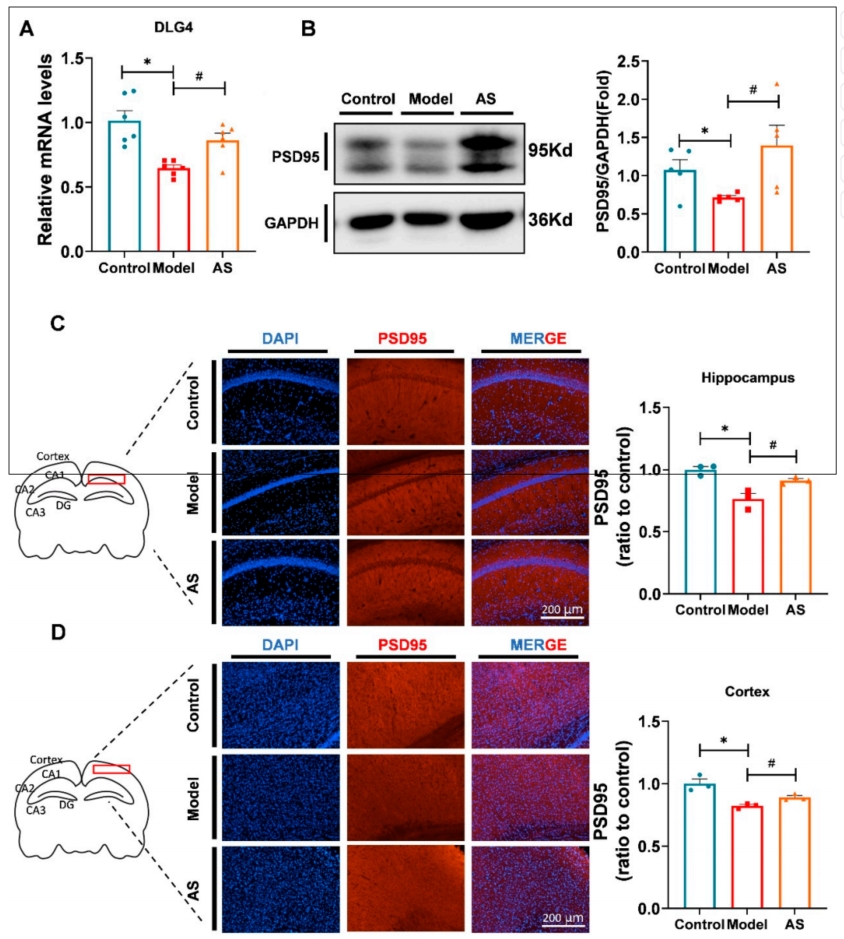
- Synaptic Repair and Function Enhancement:
- Preparatory Work:
The expression of synaptic proteins (PSD95) was measured to evaluate synaptic integrity and repair. - Experimental Procedure:
Western blotting and immunofluorescence were used to measure PSD95 protein levels in the hippocampus and cortex. - Results:
- AS treatment led to a significant upregulation of PSD95 expression in both the hippocampus and cortex.
- A significant increase in dendritic spine density was observed in AS-treated mice, indicating enhanced synaptic function and repair.
- New Findings:
AS promoted synaptic repair through increased expression of synaptic proteins, potentially reversing synaptic damage caused by AD pathology.
- Preparatory Work:
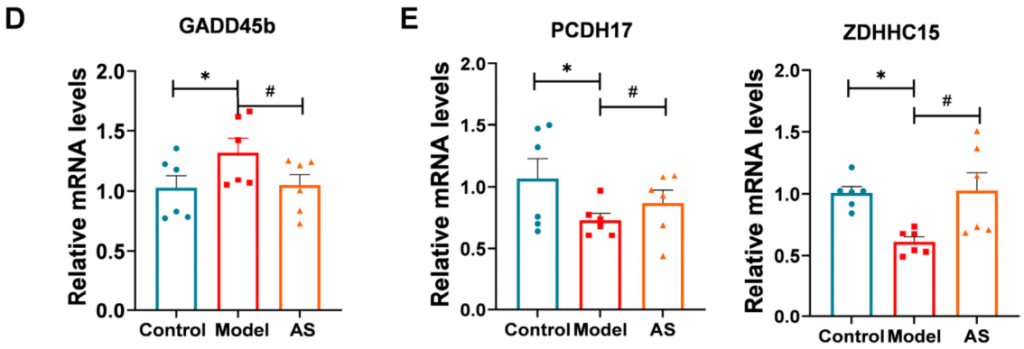
- Gene Expression Changes via RNA Sequencing:
- Preparatory Work:
RNA was isolated from brain tissues of AS-treated and model mice for sequencing analysis. - Experimental Procedure:
RNA sequencing was conducted to identify differentially expressed genes (DEGs) between the AS-treated and model groups. - Results:
- 22 DEGs were identified, including genes involved in inflammation and synaptic function (e.g., Gadd45b, Pcdh17, Zdhhc15).
- Notably, AS treatment downregulated Gadd45b expression, suggesting its impact on the p38 MAPK pathway, a critical regulator of neuroinflammation.
- New Findings:
The alteration of genes related to inflammation and synaptic function further supports the hypothesis that AS modulates critical pathways involved in AD pathology.
- Preparatory Work:
Summary of Key Findings and Implications
Significance
The study demonstrates that Asiaticoside (AS) can improve cognitive function and reduce neuroinflammation in AD mice by enhancing synaptic repair. These findings support the potential of AS as a candidate for the treatment of Alzheimer’s disease.
AS was shown to modulate critical pathways, such as the p38 MAPK pathway, which influences both inflammation and synaptic function. This dual effect positions AS as a promising therapeutic option that could address two key contributors to AD progression. This research adds to the growing evidence that targeting both neuroinflammation and synaptic dysfunction could offer a comprehensive approach to AD treatment.
Limitaitons and Future Directions
One significant limitation is the difficulty in delivering Asiaticoside (AS) across the blood-brain barrier (BBB), as it has a large molecular weight and high topological polar surface area. This could limit its bioavailability in the brain, potentially reducing its therapeutic effects. Future studies may need to explore suitable delivery systems, such as liposomes or lipid nanoparticles, to improve AS delivery to the brainclear Molecular Mechanisms.
While the study provided valuable insights into the therapeutic effects of AS, the exact molecular mechanisms underlying its ability to alleviate neuroinflammation and promote synaptic repair remain unclear. Further research is necessary to better understand how AS modulates pathways like the p38 MAPK signaling pathway and how it affects synaptic function at the molecular level.
Reference:
Liu, Sai, et al. “Asiaticoside mitigates Alzheimer’s disease pathology by attenuating inflammation and enhancing synaptic function.” International Journal of Molecular Sciences 24.15 (2023): 11976.
