Author: Tiffany
Researchers have designed an atomoxetine-loaded nanostructured lipid carrier (NLC) in situ gel, demonstrating potential for improving drug delivery to the brain via the nasal route, providing a new avenue for treating dementia.
Key Highlights
- Research Question:
Can a thermosensitive, atomoxetine-loaded nanostructured lipid carrier (NLC) in situ gel improve nose-to-brain drug delivery for dementia treatment by overcoming bioavailability and targeting limitations? - Research Difficulties:
Optimizing the formulation to overcome nasal mucociliary clearance, limited cavity volume, and enzymatic degradation at the olfactory epithelium. - Key Findings:
The optimized gel achieved 1.59-fold higher bioavailability and 51.91% improved brain targeting, with significant cognitive improvements in dementia models. - Innovative Aspects:
Combined NLC and thermosensitive gel technology for sustained release and enhanced brain delivery via the nasal route. - Importance of the Study:
Provides a non-invasive approach to improve dementia treatment, addressing critical limitations in drug delivery and bioavailability.
Understanding Dementia and Its Challenges
Dementia is a progressive neurodegenerative syndrome affecting approximately 50 million people worldwide. Its symptoms include memory loss, impaired cognitive and executive functions, and behavioral changes, severely impacting daily life.
Vascular dementia, a subtype of dementia, results from disrupted blood flow to the brain, leading to neuronal damage. It is often associated with cardiovascular and cerebrovascular conditions.
Current therapeutic options, including pharmacological agents like donepezil, face challenges such as limited bioavailability and difficulty crossing the blood-brain barrier (BBB). Addressing these barriers is critical for advancing dementia care.
Intranasal drug delivery has emerged as a promising, non-invasive method, leveraging the nasal cavity’s direct pathways to the brain via the olfactory and trigeminal regions.
Research Objectives
This study sought to overcome the limitations of existing dementia treatments by developing a thermosensitive atomoxetine-loaded NLC in situ gel for nose-to-brain delivery. The goal was to enhance atomoxetine’s bioavailability, retention in the nasal cavity, and brain targeting efficiency.
The research team included Dibyalochan Mohanty, Omar Awad Alsaidan, Ameeduzzafar Zafar, Trishala Dodle, and others from institutions such as Anurag University, Jouf University, and Prince Sattam Bin Abdulaziz University. The study was published in Pharmaceutics in July 2023.
Step-by-Step Experimental Approach
Overview of Research Procedures
- Atomoxetine-loaded NLCs were prepared using the melt emulsification ultrasonication method.
- Optimization of the formulation was conducted using the Box–Behnken statistical design.
- The optimized NLC was incorporated into a thermosensitive in situ gel using poloxamer 407 and carbopol 934P.
- The formulation was evaluated through physicochemical characterizations, in vitro drug release, ex vivo permeation studies, and preclinical pharmacokinetic and pharmacodynamic assessments in Wistar rats.
Detailed Key Experiments and Findings
- Preparation and Characterization of NLCs:
- Preparatory Work: Stearic acid and olive oil were chosen as lipid components based on their compatibility and solubility with atomoxetine. Tween 80 was selected as a surfactant.
- Procedure: Using the melt emulsification ultrasonication method, atomoxetine-loaded NLCs were prepared. The Box–Behnken design optimized parameters such as lipid concentration, surfactant levels, and sonication time.
- Results: The optimized NLC exhibited a particle size of 108 nm, a zeta potential of -42.3 mV, and an entrapment efficiency of 84.12%. These characteristics ensured stability and effective drug delivery potential.
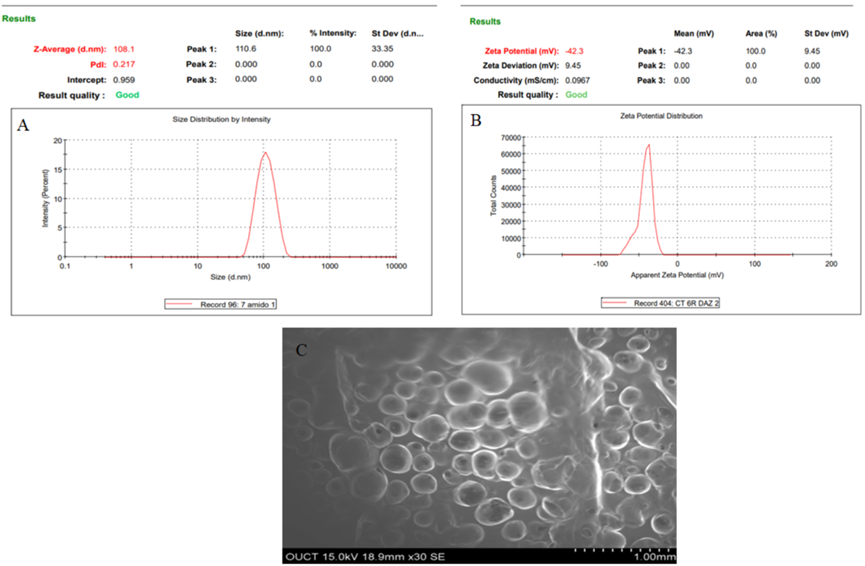
Development of Thermosensitive in Situ Gel:
- Preparatory Work: Poloxamer 407 and carbopol 934P were selected for their ability to form temperature-sensitive gels. Viscosity and gelling temperature were evaluated to refine the formulation.
- Procedure: The optimized NLC (AXT-NLC13) was incorporated into the gel base. A series of formulations were prepared and assessed for physicochemical properties.
- Results: The finalized gel formulation (AXT-NLC13-G4) gelled at 28–34°C and provided sustained drug release (92.89% over 12 hours), surpassing pure atomoxetine (95.47% in 4 hours). The gel’s viscosity at 37°C was measured at 2532 ± 18 cps, demonstrating suitability for nasal administration.
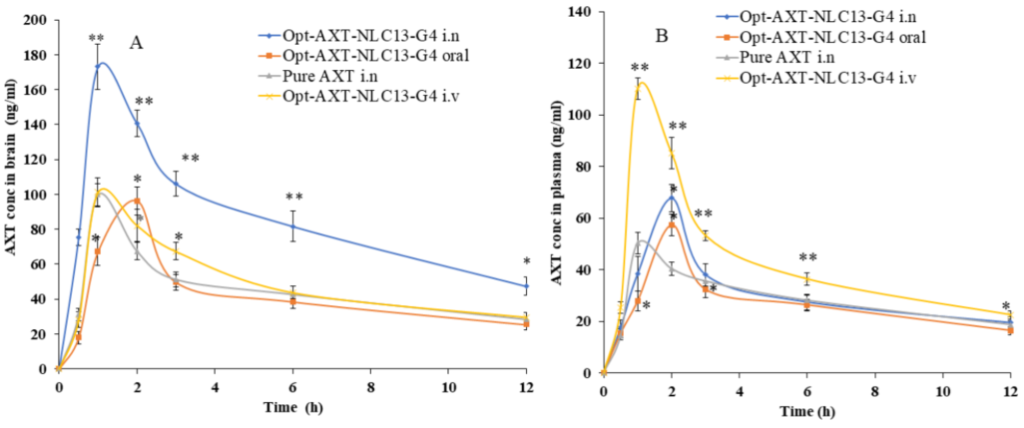
- Pharmacokinetic and Pharmacodynamic Studies:
- Preparatory Work: Wistar rats were utilized to examine bioavailability and brain targeting efficiency of the in situ gel.
- Procedure: The gel was administered intranasally, and plasma and brain samples were collected over 12 hours. Cognitive effects were assessed using the Morris Water Maze to evaluate spatial learning and memory.
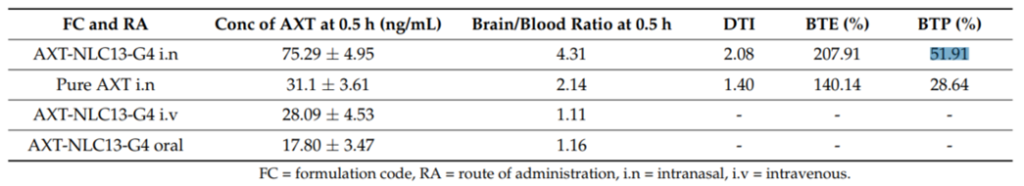
- Results: The intranasal gel showed a 1.59-fold increase in bioavailability and a 51.91% improvement in brain transport compared to pure atomoxetine. Behavioral studies revealed significant cognitive improvements in comparison to the control and standard treatments.
Key Outcomes and Implications
This study developed an atomoxetine-loaded NLC in situ gel, achieving enhanced delivery to the brain through the nasal route. Key findings included:
- Sustained drug release and extended retention within the nasal cavity.
- Improved bioavailability and efficient brain targeting.
- Demonstrable cognitive benefits in preclinical dementia models.
The innovation of this research lies in the integration of NLC technology with thermosensitive gel formulations, addressing critical challenges in drug delivery and dementia treatment.
The study also highlights several limitations, including challenges with nasal retention due to mucociliary clearance, limited volume capacity of the nasal cavity, and enzymatic degradation at the olfactory epithelium, which can affect drug delivery efficiency. Additionally, the anatomical differences between preclinical animal models and humans may lead to variations in outcomes, emphasizing the need for advanced predictive models. Furthermore, while the nanogel system offers promise, issues such as initial burst release and slow responsiveness to stimuli require further optimization. These factors underline areas for improvement in future research to enhance the formulation’s clinical applicability.
Reference:
Mohanty, Dibyalochan, et al. “Development of atomoxetine-loaded NLC in situ gel for nose-to-brain delivery: optimization, in vitro, and preclinical evaluation.” Pharmaceutics 15.7 (2023): 1985.
