Editor: Sarah
The Growing Challenge of Antibiotic Resistance
Antibiotic resistance is one of the most pressing global health challenges, driven by the overuse and misuse of antibiotics and the adaptive capabilities of bacteria. This phenomenon is reducing the effectiveness of traditional antibiotics, leaving clinicians with limited options to treat infections. As the number of newly developed antibiotics continues to decline, researchers are turning to innovative technologies, such as nanotechnology, to address this urgent issue.
A recent study introduces a novel solution: a vanadium-based MXene nanoplatform (V2N) designed to combat antibiotic-resistant bacteria. By integrating dual enzyme-like activities with photothermal effects, this nanoplatform shows potential in killing bacteria, disrupting biofilms, and accelerating wound healing.
Why Biofilms Are So Problematic
Bacterial biofilms are complex structures composed of bacteria encased in extracellular polymeric substances (EPS), which act as a physical and chemical barrier. These structures shield bacteria from antibiotics and immune responses, making infections persistent and recurrent. Biofilms not only prevent drug penetration but also foster a microenvironment where bacteria thrive and adapt, increasing resistance to treatments.
Traditional antibiotics often fail to penetrate these biofilms, necessitating new approaches. One promising avenue is the use of nanozymes, which mimic the catalytic activity of natural enzymes. Nanozymes generate reactive oxygen species (ROS) to kill bacteria and degrade biofilms without encouraging antibiotic resistance. However, achieving the desired catalytic efficiency in complex infection environments has been a challenge.
A Multi-Functional Approach: The V2N Nanoplatform
This study highlights the development of a valence-switchable vanadium-based MXene (V2N) nanoplatform that addresses these challenges through three key mechanisms:
- Dual Enzyme-Like Activities: V2N exhibits oxidase-like (OXD) and peroxidase-like (POD) activities, producing ROS that effectively degrade biofilms and kill bacteria. These activities are optimized for weakly acidic conditions, mimicking the microenvironment of bacterial biofilms.
- Photothermal Enhancement: When exposed to near-infrared (NIR-II) light (1064 nm), V2N generates localized heat, significantly boosting its catalytic activity. This combined effect drastically reduces bacterial survival rates, with experimental results showing bacterial viability as low as 4.1% for Staphylococcus aureus and 2.5% for Streptococcus mutans.
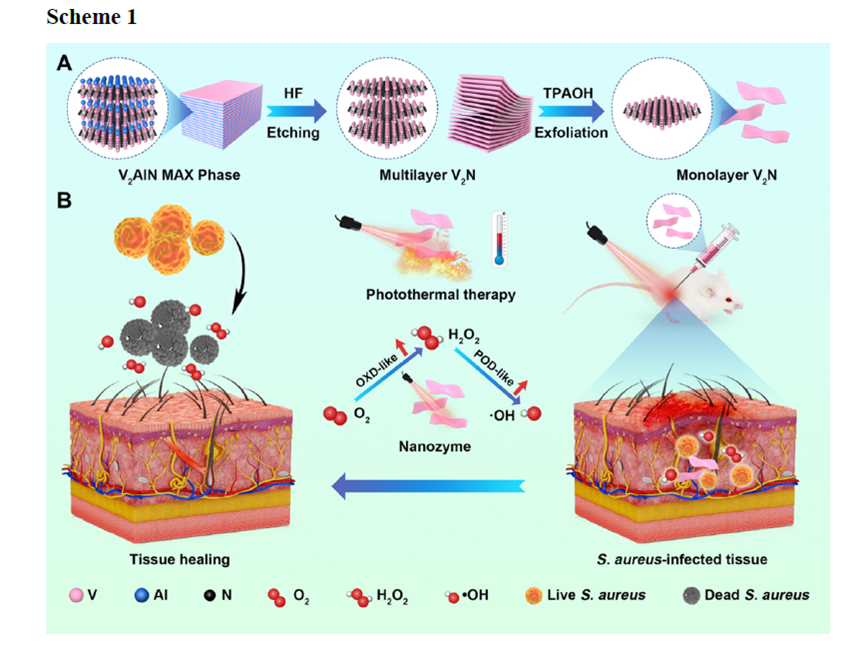
Scheme 1. Schematic illustration of (A) the preparation of monolayer V2N and (B) 1064 nm laser irradiation enhanced dual enzyme-like catalytic activities of V2N for promoting the healing of S. aureus-infected abscesses in vivo.
- In Vivo Efficacy: In a mouse abscess model, the V2N nanoplatform demonstrated its ability to clear infections and promote wound healing. After 10 days of treatment, the wound area in the V2N-treated group was reduced to just 5.5%, compared to 45.3% in the control group. Histological analysis confirmed reduced inflammation and enhanced tissue regeneration.
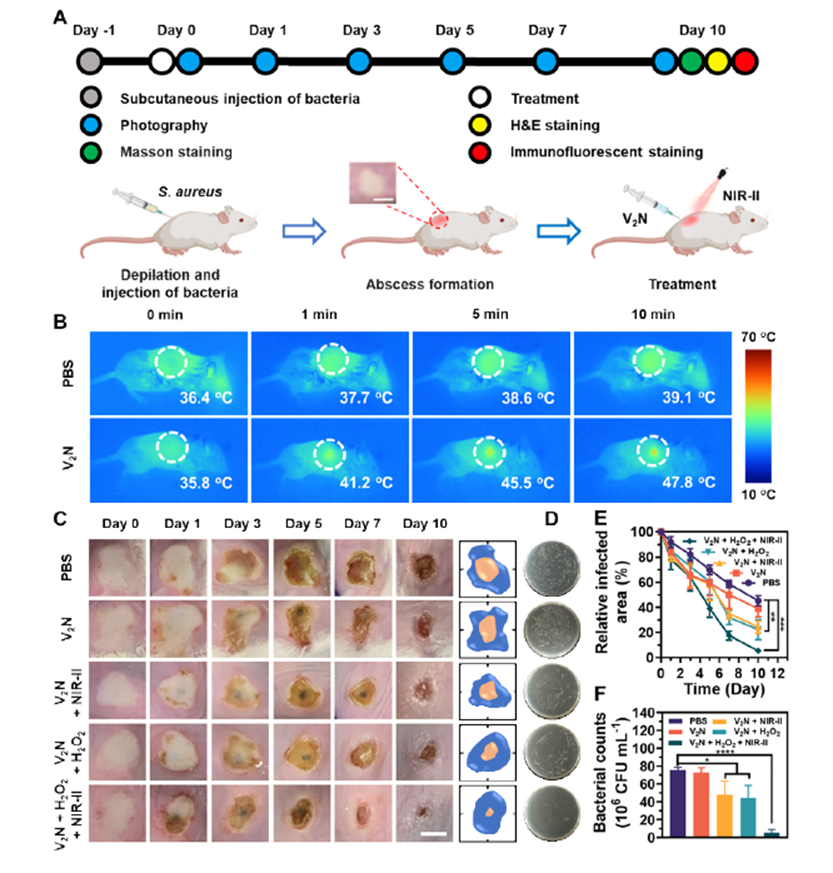
Figure 1. Anti-infective Therapy of V2N in Vivo with Subcutaneous Abscess Model.
Comprehensive Evaluation: From Synthesis to Safety
The researchers synthesized V2N using a modified chemical exfoliation method, ensuring the production of monolayer nanosheets with desirable physical and chemical properties. Advanced characterization techniques validated its structure and performance. In vitro experiments showed its effectiveness in destroying biofilms and planktonic bacteria, while in vivo testing demonstrated its ability to accelerate wound healing.
Safety assessments revealed no significant toxicity. Biosafety evaluations, including blood tests and organ histology, confirmed that V2N does not harm major organs or cause adverse effects during treatment, highlighting its potential for clinical application.
Implications for Healthcare and Infectious Disease Management
The development of the V2N nanoplatform offers promising implications for tackling antibiotic resistance and improving treatment outcomes for infectious diseases:
- An Alternative to Traditional Antibiotics: V2N kills bacteria through a non-antibiotic mechanism, reducing the risk of resistance development.
- Enhanced Wound Healing: Its dual functionality not only clears infections but also promotes tissue regeneration, making it particularly useful for treating chronic wounds and abscesses.
- Cost-Effective Treatment: By reducing reliance on traditional antibiotics, this nanoplatform could help lower healthcare costs associated with drug-resistant infections.
- Broader Impact: The technology aligns with global efforts to combat antimicrobial resistance, benefiting researchers, clinicians, and policymakers alike.
Real-World Applications and Challenges Ahead
The successful application of V2N in preclinical models paves the way for its translation into clinical practice. Its ability to disrupt biofilms and clear infections without significant side effects is a major step forward in the fight against resistant bacteria. The photothermal enhancement of catalytic activity is particularly significant, as it demonstrates the potential for combining nanotechnology with light-based therapies.
However, several challenges remain. Scaling up production, long-term safety studies, and clinical trials are essential steps to ensure the widespread adoption of this technology. The interaction of V2N with different biological environments and its performance in diverse infection scenarios need further investigation.
Reference
Sun, Xiaoshuai, et al. “Valence-Switchable and Biocatalytic Vanadium-Based MXene Nanoplatform with Photothermal-Enhanced Dual Enzyme-Like Activities for Anti-Infective Therapy.” SSRN Electronic Journal, 2025, https://ssrn.com/abstract=4156471
