Author: Tiffany
A recent study highlights the effectiveness of GSNO-loaded nanoparticles in controlling blood pressure and preserving kidney function in young rats with CKD, offering new insights for pediatric care.
Key Highlights
- Research Question:
Can GSNO-loaded Cu/ZIF-8 nanoparticles effectively deliver nitric oxide (NO) to reduce hypertension and improve kidney function in a pediatric CKD model? - Research Difficulties:
Developing a stable NO delivery system with sustained release while avoiding oxidative stress and ensuring safety in CKD conditions. - Key Findings:
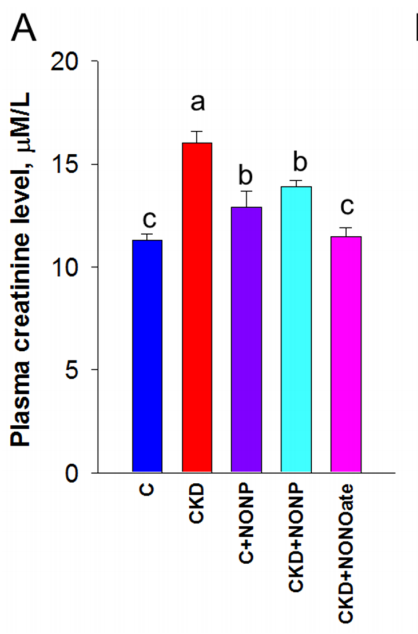
GSNO-loaded nanoparticles reduced blood pressure, improved kidney function (plasma creatinine: 16 ± 0.6 µM to near-control levels of 11.3 ± 0.3 µM), decreased oxidative stress (8-OHdG levels), and downregulated key RAS components (renin, ACE1, AT1R). - Innovative Aspects:
First use of GSNO-loaded Cu/ZIF-8 nanoparticles for stable and sustained NO delivery in CKD treatment. - Importance of the Study:
Provides a novel therapeutic approach to address hypertension and kidney dysfunction in pediatric CKD, a critical unmet need in current medical practice.
Understanding the Impact of CKD and Hypertension in Children
Chronic kidney disease (CKD) is a significant health issue, particularly for children, where it frequently manifests with hypertension as a major complication. Research indicates that over half of pediatric CKD patients experience elevated blood pressure, even in the disease’s early stages. This condition substantially increases the likelihood of cardiovascular issues and poses challenges for management, as many children fail to achieve adequate blood pressure control despite using multiple antihypertensive medications.
Nitric oxide (NO) plays a critical role in maintaining blood pressure and kidney health, but its deficiency is a central factor in the pathogenesis of CKD and related hypertension. Causes of NO deficiency include suppressed synthesis, oxidative stress, and inhibition by endogenous compounds like asymmetric dimethylarginine (ADMA). Existing NO-targeted therapies face limitations such as rapid degradation of NO donors and suboptimal delivery mechanisms, creating a need for improved strategies. Nanoparticle-based NO delivery systems have been proposed as a potential solution, promising enhanced stability and sustained release of NO, which may offer benefits for managing CKD-induced hypertension.
Objectives and Scope of the Research
This study sought to evaluate the efficacy of GSNO-loaded nanoparticles as a novel NO delivery system in addressing hypertension and kidney dysfunction in young rats with CKD. Researchers aimed to:
- Assess the effectiveness of GSNO-loaded Cu/ZIF-8 nanoparticles in delivering NO and mitigating CKD-induced hypertension.
- Compare the therapeutic effects of GSNO-loaded nanoparticles with the established NO donor DETA NONOate.
- Investigate the impact of these treatments on kidney function, oxidative stress, and renin-angiotensin system (RAS) activity.
The study, conducted by a multidisciplinary team from institutions such as Kaohsiung Chang Gung Memorial Hospital and National Cheng Kung University, Taiwan, was published in Antioxidants in February 2023.
Experimental Approach and Observations
Outline of Research Process
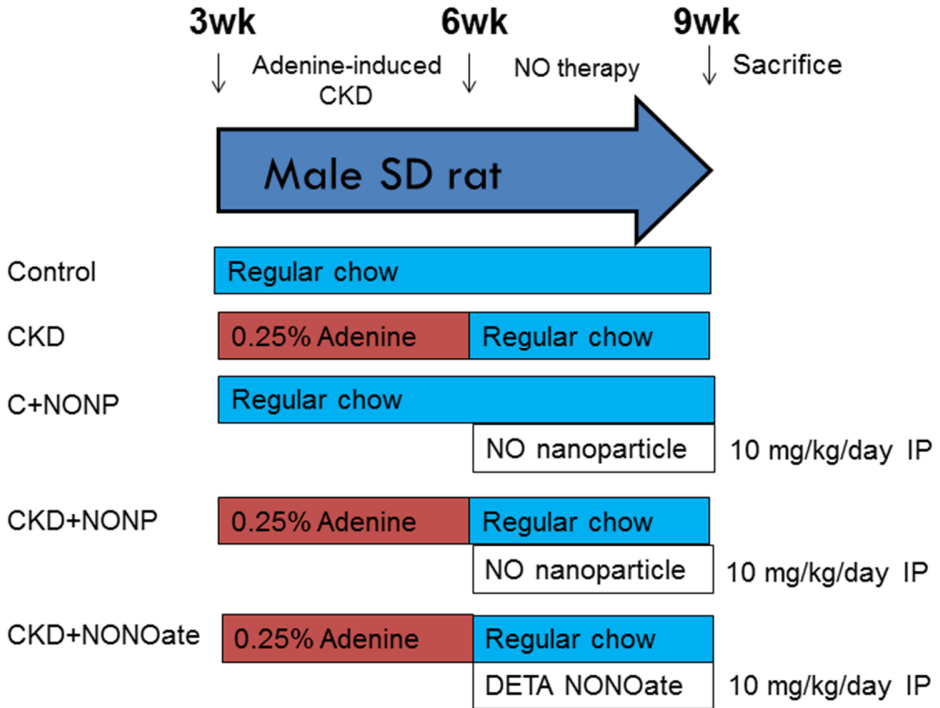
- Induction of CKD: CKD was induced in male Sprague-Dawley rats through a diet supplemented with 0.25% adenine for three weeks.
- Grouping and Treatments: Rats were divided into five groups: healthy controls, CKD, CKD treated with GSNO-loaded nanoparticles, CKD treated with DETA NONOate, and healthy rats treated with NO nanoparticles.
- Treatment Administration: Treatments were delivered via intraperitoneal injection at a dosage of 10 mg/kg/day for three weeks.
- Outcome Measurements: Researchers evaluated blood pressure, plasma creatinine, oxidative stress markers, and RAS components to assess treatment effects.
Key Experiments and Findings
1. Development and Characterization of GSNO-Loaded Nanoparticles
- Preparatory Steps: GSNO-loaded Cu/ZIF-8 nanoparticles were synthesized using a mixture of ZnCl2, Cu(NO3)2, 2-Methylimidazole, and polyvinyl alcohol. The reaction mixture was stirred under nitrogen flow, centrifuged, washed, and dried.
- Results: Microscopy confirmed the nanoparticles’ structure, showing a rhombic dodecahedron shape with an average side length of 381 ± 17.6 nm. The GSNO loading efficiency was approximately 24.7 ± 3.2 wt%. Stability tests revealed that the Cu/ZIF-8 framework effectively protected GSNO, facilitating prolonged NO release.
2. Effects on Blood Pressure and Kidney Function
- Preparatory Steps: Young rats fed with adenine to induce CKD were randomly assigned to different treatment groups.
- Experimental Procedure: Blood pressure was measured biweekly using a tail-cuff method, while plasma creatinine levels were analyzed with high-performance liquid chromatography (HPLC).
- Results: CKD rats showed significant blood pressure elevation, which was reduced with GSNO-loaded nanoparticles and DETA NONOate treatments. Plasma creatinine levels increased in CKD rats (16 ± 0.6 µM vs. 11.3 ± 0.3 µM in controls, p < 0.05). Both treatments improved kidney function, though DETA NONOate demonstrated slightly greater efficacy.
3. Assessment of Oxidative Stress Markers
- Preparatory Steps: Immunostaining was performed on kidney tissues to detect 8-hydroxydeoxyguanosine (8-OHdG), a marker of oxidative DNA damage.
- Results: CKD-induced oxidative stress was evident from intense 8-OHdG staining in kidney glomeruli and tubules. Both GSNO-loaded nanoparticles and DETA NONOate treatments reduced oxidative stress, with significantly lower 8-OHdG staining observed in treated groups.
4. Modulation of the Renin-Angiotensin System (RAS)
- Preparatory Steps: Quantitative PCR was conducted to evaluate renal mRNA expression of key RAS components, including renin, ACE1, ACE2, and AT1R.
- Results: CKD rats exhibited heightened RAS activity, as reflected by increased renin, ACE1, and AT1R expression. GSNO-loaded nanoparticles effectively reduced these levels, aligning with their antihypertensive effect. DETA NONOate similarly downregulated AT1R expression, demonstrating comparable efficacy in modulating the RAS.
Study Implications and Future Directions
This research demonstrated that GSNO-loaded Cu/ZIF-8 nanoparticles provide a stable and sustained NO delivery system that reduces blood pressure and oxidative stress while improving kidney function. Both GSNO-loaded nanoparticles and DETA NONOate showed similar antihypertensive effects, with the nanoparticles offering additional benefits in reducing RAS activity and restoring NO bioavailability.
The use of Cu/ZIF-8 nanoparticles to stabilize and deliver GSNO addresses key limitations of traditional NO therapies, such as short half-life and inadequate delivery. This approach combines effective blood pressure management with kidney protection, offering a promising solution for pediatric CKD patients. The findings support further exploration of nanoparticle-based NO delivery systems as a viable therapeutic strategy.
Reference:
Tain, You-Lin, et al. “Anti-Hypertensive Property of an NO Nanoparticle in an Adenine-Induced Chronic Kidney Disease Young Rat Model.” Antioxidants 12.2 (2023): 513.
