Author: Tiffany
Scientists have designed a nanotechnology-based approach using extracellular vesicles encapsulating CHIP to address renal fibrosis, offering a potential advancement in chronic kidney disease treatment.
Key Highlights:
- Research Question: Can engineered extracellular vesicles (EVs) encapsulating CHIP, combined with superparamagnetic iron oxide nanoparticles (SPION), enhance targeted delivery to renal tissues and address the limitations of current therapies for renal interstitial fibrosis (RIF), including poor renal targeting and insufficient efficacy?
- Research Difficulties:
- Achieving stable expression of CHIP in extracellular vesicles while maintaining its bioactivity during delivery.
- Ensuring efficient renal targeting of EVs through SPION modification and magnetic field guidance.
- Overcoming the rapid clearance of EVs from circulation and ensuring selective accumulation in injured renal tissues.
- Key Findings [Highlighting Impact and Novelty]:
- SPION-engineered CHIP-loaded EVs significantly reduced fibrosis markers (α-SMA, Collagen I, Fibronectin) and inflammatory cytokines (IL-6, IL-1β, TNF-α) in UUO and DKD animal models.
- Renal tubular injury scores improved from 70% in untreated UUO models to 18% in SPION-EVs-CHIP-treated groups.
- Demonstrated enhanced renal targeting under magnetic guidance, achieving higher CHIP delivery and Smad2/3 degradation compared to unmodified EVs.
- Innovative Aspects:
- First study to combine SPION with CHIP-loaded EVs to create a magnetic field-guided nanotherapeutic system.
- Novel use of CHIP to promote Smad2/3 ubiquitination and degradation, directly targeting a key pathway in fibrosis progression.
- Integration of nanotechnology and protein degradation strategies for enhanced therapeutic efficacy.
Understanding Renal Fibrosis and Current Challenges
Overview of Renal Fibrosis:
Renal interstitial fibrosis (RIF) represents a key pathological feature of chronic kidney disease (CKD). It involves an accumulation of extracellular matrix components, leading to impaired renal function and tissue scarring, which may ultimately progress to end-stage renal failure. CKD affects around 15% of the global population and continues to grow in prevalence due to aging demographics and evolving lifestyles.
Symptoms and Progression
Patients with CKD and RIF often present with proteinuria, hypertension, and diminished glomerular filtration rates, signaling progressive kidney damage. Chronic inflammation, epithelial cell injury, and fibroblast activation are central to its pathophysiology.
Current Therapeutic Options and Limitations
Current treatments aim to slow fibrosis progression, targeting inflammatory and fibrotic pathways using therapies such as ACE inhibitors and angiotensin receptor blockers. However, these strategies fail to reverse fibrosis and can carry limitations, including suboptimal renal targeting and potential adverse effects.
Goals and Objectives of the Research
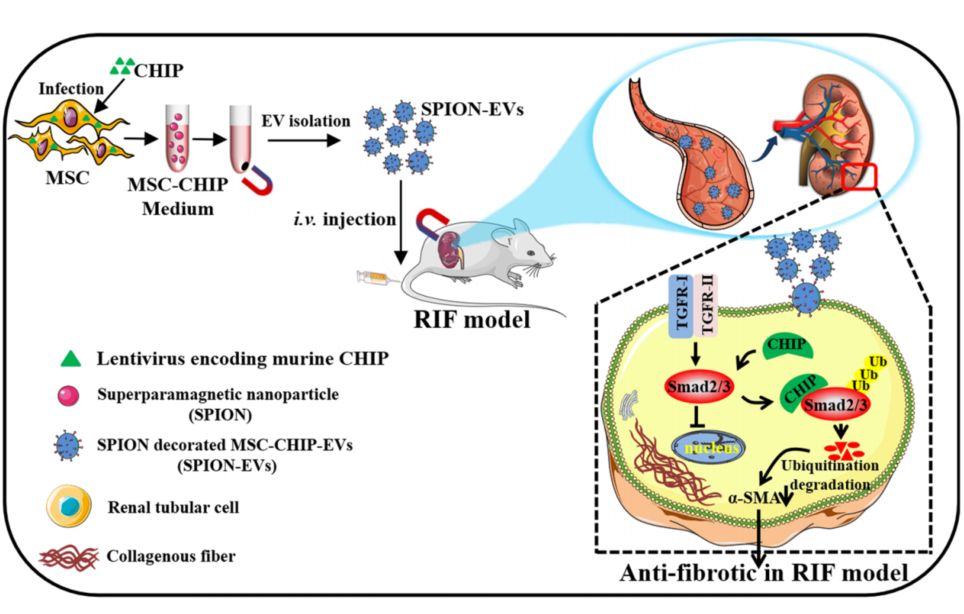
This study seeks to address the limitations of existing therapies by developing engineered extracellular vesicles (EVs) enriched with CHIP, a protein known to degrade Smad2/3—a critical regulator of fibrosis. To enhance renal targeting, superparamagnetic iron oxide nanoparticles (SPION) were incorporated into the EVs, enabling their localization to injured kidneys under a magnetic field. Conducted by Cheng Ji, Jiahui Zhang, Linru Shi, and colleagues at Jiangsu University, China, the study was published in NPJ Regenerative Medicine in January 2024.
Experimental Design and Key Results
Sequential Outline of Experimental Process
- Mesenchymal stem cells (MSCs) were isolated from human umbilical cords and transfected with lentivirus encoding CHIP.
- Extracellular vesicles (EVs) were extracted and purified from the MSCs through ultracentrifugation.
- SPION nanoparticles were attached to the EVs to provide magnetic targeting capabilities.
- The engineered SPION-EVs-CHIP were tested in vitro on renal tubular cells (NRK-52E).
- SPION-EVs-CHIP efficacy was assessed in two animal models: unilateral ureteral obstruction (UUO) and diabetic kidney disease (DKD).
- Biochemical and histological methods were employed to evaluate renal function, fibrosis markers, and inflammation.
Detailed Insights into Key Experiments
- Preparation and Characterization of Engineered EVs:
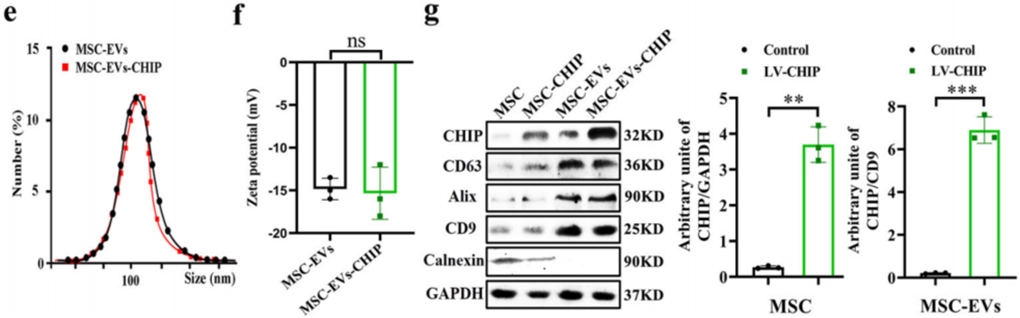
- Preparatory Work: MSCs were transfected with lentivirus encoding CHIP to generate high CHIP-expressing cells. Transfection success was confirmed via western blotting and confocal microscopy, showing CHIP localization in the cytoplasm. EVs were isolated from the MSC culture supernatant using ultracentrifugation and characterized by transmission electron microscopy (TEM).
- Results: MSC-EVs-CHIP exhibited an average particle size of 108 ± 10.6 nm with a zeta potential of approximately -15mV. Western blot analysis validated high CHIP expression in the EVs.
- In Vitro Testing of SPION-EVs-CHIP on Renal Tubular Cells:
- Experimental Procedure: NRK-52E cells were incubated with GFP-tagged MSC-EVs-CHIP, with TGF-β1 applied to induce fibrosis-like changes. Confocal imaging and western blotting were utilized to assess Smad2/3 degradation and expression of fibrosis markers.
- Results: Internalization of EVs by NRK-52E cells was confirmed through GFP fluorescence. CHIP co-localized with Smad2/3, leading to its ubiquitination and degradation. Reduced nuclear accumulation of Smad2/3 and decreased α-SMA levels were observed.
- Findings: MSC-EVs-CHIP demonstrated efficacy in mitigating TGF-β1-induced fibrosis-like changes in renal tubular cells.
- Efficacy in the UUO Rat Model:
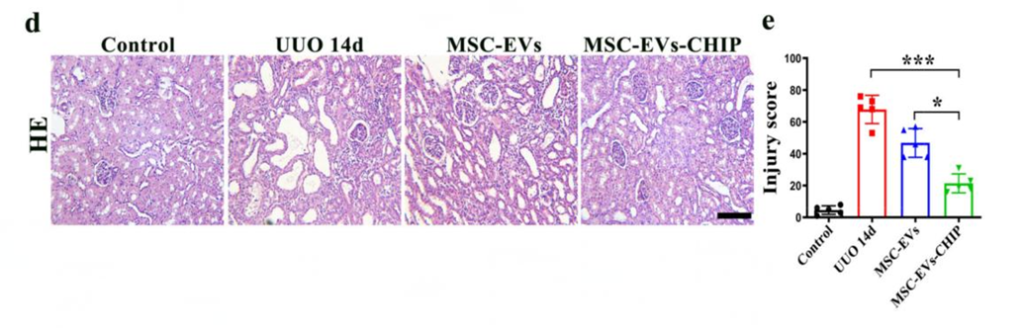
- Preparatory Work: UUO rats were treated intravenously with SPION-EVs-CHIP, MSC-EVs, or PBS. A magnetic field was employed to direct SPION-EVs to injured renal tissue.
- Experimental Procedure: CM-DIR labeling enabled tracking of EV distribution. Histological assessments (HE, Sirius Red, and Masson staining) and western blotting were performed to measure fibrosis and inflammation.
- Results: SPION-EVs-CHIP preferentially accumulated in the kidney under magnetic guidance, reducing fibrosis markers (α-SMA, Collagen I, and Fibronectin) and inflammatory cytokines (IL-6, IL-1β, TNF-α). Tubular injury scores improved significantly, decreasing from 70% in untreated rats to 18% in SPION-EVs-CHIP-treated groups.
- Findings: Enhanced targeting and therapeutic effects were achieved with SPION-EVs-CHIP compared to MSC-EVs alone.
- Efficacy in the DKD Rat Model:
- Preparatory Work: DKD was induced in rats through a high-fat diet and streptozotocin (STZ) injection. SPION-EVs-CHIP were administered intravenously.
- Experimental Procedure: Histological staining and immunofluorescence were utilized to evaluate fibrosis severity and protein expression in renal tissues.
- Results: SPION-EVs-CHIP treatment significantly reduced tubular atrophy, fibrosis area (quantified through Sirius Red and Masson staining), and levels of fibrosis markers (α-SMA, Collagen I, Fibronectin). Nuclear Smad2/3 levels were also lowered.
- Findings: These results confirmed that SPION-EVs-CHIP could effectively attenuate fibrosis and inflammation in DKD rats.
Advancing Nanotechnology for Renal Therapy
This study demonstrates that SPION-engineered CHIP-loaded MSC-EVs effectively target injured renal tissues under a magnetic field, promoting Smad2/3 degradation and alleviating fibrosis. In both UUO and DKD animal models, this approach significantly reduced fibrosis markers (α-SMA, Collagen I, Fibronectin), cytokines (IL-6, IL-1β, TNF-α), and tubular injury scores (from 70% to 18% in UUO models), while improving renal function and structure.
This research is the first to integrate SPION with CHIP-loaded MSC-EVs, establishing a magnetic targeting nanotherapy system. The innovation enhances CHIP stability and renal enrichment, paving the way for a novel therapeutic platform that combines molecular degradation strategies with advanced nanotechnology for renal fibrosis treatment.
Importance of the Study:
- Addresses a critical need for effective and targeted therapies for chronic kidney disease (CKD) by overcoming limitations of current antifibrotic treatments.
- Provides a platform for future development of nanotherapeutics in renal and other fibrotic diseases.
- Paves the way for clinical applications that could improve patient outcomes by reducing fibrosis progression and preserving renal function.
Reference:
Ji, Cheng, et al. “Engineered extracellular vesicle-encapsulated CHIP as novel nanotherapeutics for treatment of renal fibrosis.” NPJ Regenerative Medicine 9.1 (2024): 3.
