Editor: Nina
Key Preview
Research Question
- How effectively can newly developed mRNA vaccines encoding fusion proteins of MPXV antigens protect against lethal VACV infection?
Research Design and Strategy
- Preclinical experimental design using female Balb/c mice as models.
- Comparison of three distinct mRNA vaccine candidates: VGPox 1, VGPox 2, and VGPox 3, each designed to encode specific MPXV antigens.
- Targeted immunogenicity and efficacy evaluation based on the antigen combinations A35R and M1R.
Method
- Lipid nanoparticle (LNP) encapsulation was utilized for mRNA delivery.
- Vaccination outcomes were evaluated through immune markers like antibody titers and neutralization assays.
- Post-vaccination, the protective efficacy was assessed by challenging the mice with lethal doses of VACV.
Key Results
- All vaccines induced anti-A35R antibodies by day 7 post-vaccination.
- VGPox 1 and VGPox 2 demonstrated higher anti-M1R antibody levels compared to VGPox 3.
- Complete protection against VACV challenge was observed for all vaccine groups.
- VGPox 2 showed superior passive protection, highlighting its long-term immunological benefits.
Significance of the Research
- This study demonstrates the potential of mRNA vaccines as a viable alternative to traditional orthopoxvirus vaccines.
- Rapid immunogenicity and sustained protective efficacy indicate the utility of mRNA platforms in addressing emerging orthopoxvirus threats.
Introduction
The emergence of mpox has highlighted an urgent need for effective vaccination strategies. Existing vaccines, such as ACAM2000 and JYNNEOS, have raised concerns related to safety and supply limitations. Given the recent outbreaks and the World Health Organization’s declaration of a global health emergency, developing an effective and safe vaccine is paramount. mRNA technology, proven effective against SARS-CoV-2, offers a promising alternative. This study aims to address the challenges of current vaccination methods by developing innovative mRNA vaccines that encode fusion proteins of MPXV antigens, specifically A35R and M1R.
Research Team and Objective
The research team, comprising Fujun Hou, Yuntao Zhang, Xiaohu Liu, Yanal M Murad, Jiang Xu, Zhibin Yu, Xianwu Hua, Yingying Song, Jun Ding, Hongwei Huang, Ronghua Zhao, William Jia, and Xiaoming Yang, conducted this study between December 2022 and September 2023 at Shanghai Virogin Biotech Co. Ltd. The paper titled “mRNA vaccines encoding fusion proteins of monkeypox virus antigens protect mice from vaccinia virus challenge” was published in Nature Communications. The objective of the research was to evaluate the immunogenicity and protective efficacy of novel mRNA vaccine candidates against MPXV.
Experimental Process
1. mRNA Design and Protein Expression
- Key Steps: Four codon-optimized mRNA constructs encoding different MPXV antigens (A35R, M1R, and their fusion forms) were synthesized.
- Two fusion forms included SP-A35R IECD-M1R (VGPox 1) and SP-A35R sECD-M1R (VGPox 2).
- A mixture of individual mRNAs encoding full-length A35R and M1R comprised VGPox 3.
- Expression of these proteins was validated via Western blot analysis.
- Result and Key Data: SP-A35R sECD-M1R achieved the highest expression levels.
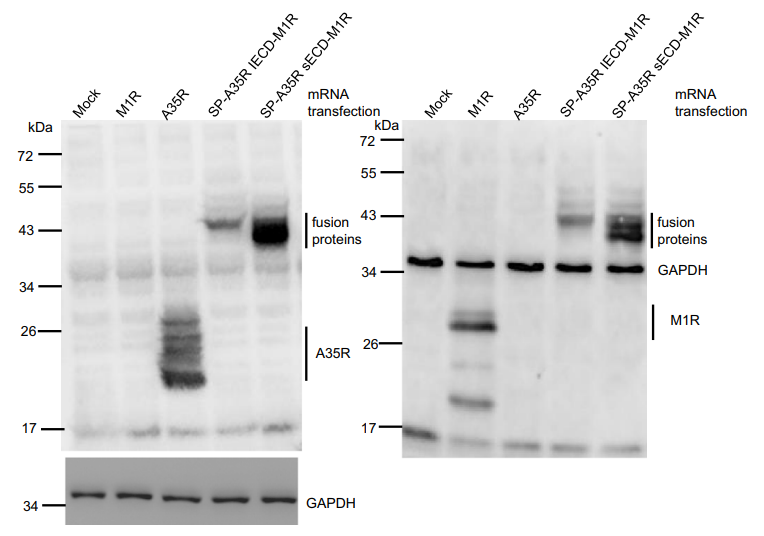
Figure 1. Protein expression measured by Western blotting. Each mRNA with 800 ng was transfected into 293 T cells, and then at 16 h posttransfection, the cell lysates were loaded into SDS-PAGE gels for Western blot analysis. Left panel: Western blot analysis with anti-A35R antibody. Right panel: Western blot analysis with anti-M1R and anti-GAPDH antibodies. The immune-blot results were repeated three times.
- Significance of the Result: The fusion design enhanced antigen expression, crucial for effective immune response.
- Key Innovation: Incorporation of a signal peptide for improved immunogenicity of M1R.
2. Vaccine Administration and Immunogenicity
- Key Steps: Female Balb/c mice received intramuscular injections of 10 µg of each mRNA vaccine. Blood and spleen samples were collected post-vaccination to assess immune responses.
- Antibody levels (A35R and M1R-specific) and neutralization capacities were measured via ELISA and plaque reduction neutralization tests (PRNT).
- Cellular immunity was assessed by flow cytometry for CD4+ and CD8+ T-cell responses.
- Result and Key Data:
- Robust anti-A35R antibodies detected in all vaccines; VGPox 1 and VGPox 2 elicited strong anti-M1R responses by day 7.
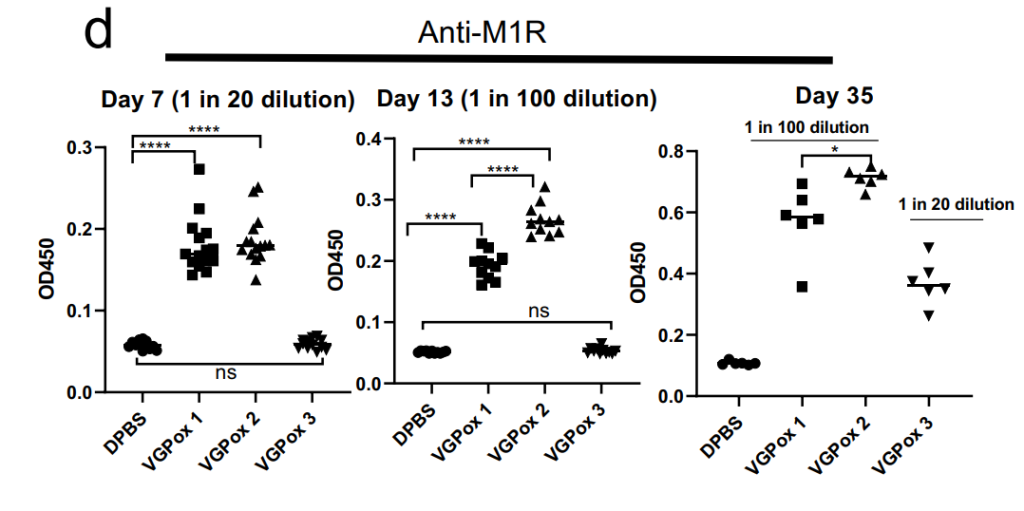
Figure 2. M1R-specific antibodies. The sera were tested for M1R-specific antibodies using ELISA against recombinant M1R protein. n = 12 to 15 for day 7 and day 13 and n = 6 for day 35 biologically independent mice.
- VGPox 1 and 2 showed superior neutralizing capacities compared to VGPox 3.
- Significance of the Result: Antibody production and neutralization indicate the effectiveness of these vaccines in priming both humoral and cellular immunity.
- Key Innovation: Fusion protein design successfully induced dual antigen-specific immunity.
3. Virus Challenge and Protection Assessment
- Key Steps: Mice were challenged intranasally with a lethal dose (1×10^6 PFU) of VACV-WR.
- Body weight and clinical symptoms were monitored to evaluate disease severity.
- Lung viral loads were measured using plaque assays to determine viral clearance.
- Result and Key Data:
- No significant weight loss or abnormalities in vaccinated groups; complete viral clearance in the lungs by day 9.
- Control groups experienced severe weight loss, with two mice succumbing to infection.
Figure 3.Protection of mRNA vaccination for virus challenge. a Virus-challenge protection. At day 36, mice were intranasally challenged with a lethal dose (1 × 106 PFU) of WR vaccinia virus. The body weight of each mouse was examined daily post virus challenge, and the changes were calculated and compared with the initial weight. n = 5 biologically independent mice. b Viral load in the lungs. The lungs were collected on day 4 in the DPBS group or on day 9 post virus inoculation in other groups. Virus titers were examined by plaque assay. n = 5 biologically independent mice.
- Significance of the Result: Demonstrates the complete protective efficacy of mRNA vaccines against lethal VACV challenge.
- Key Innovation: mRNA vaccines provided nearly sterilizing immunity within a short time frame.
4. Long-term Immunity and Passive Protection
- Key Steps:
- Vaccines were tested for long-term immunity by measuring antibody levels monthly for five months.
- Sera from vaccinated mice were transferred to naïve mice to assess passive protection.
- Result and Key Data:
- Anti-A35R antibodies gradually decreased, while anti-M1R antibodies remained stable in VGPox 1 and VGPox 2 groups.
- Passive protection tests showed superior efficacy for VGPox 2 sera in protecting naïve mice.
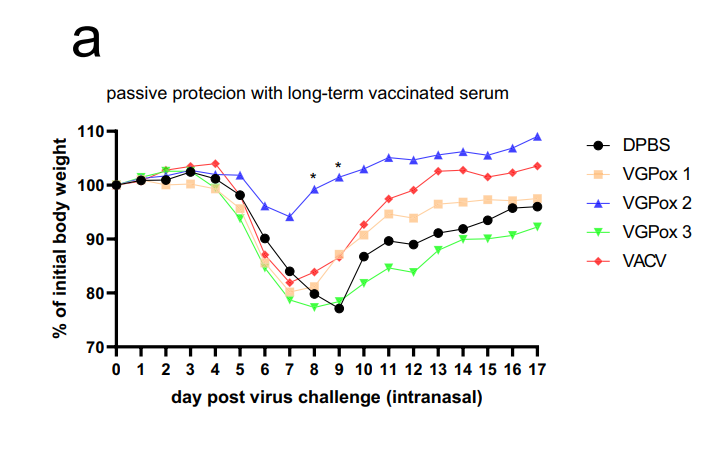
Figure 4.Passive protection by the long-term immunity sera. Naïve mice were intravenously injected with 100 µL of sera obtained from the long-term immunity group, which contained an equal-volume mixture of sera collected from months 1–4 post vaccination. The following day, the mice were intranasally challenged with 1 × 105 PFU VACV-WR. The mean body weight change post virus infection.
- Significance of the Result: Highlights the durability of immunity and potential for vaccine-induced transferable protection.
- Key Innovation: Use of passive protection assay as an indicator of serum efficacy.
5. Single-dose Vaccination Efficacy
- Key Steps:
- A single-dose vaccination model was tested to assess rapid protective immunity.
- Vaccinated mice were challenged with VACV after 7 days.
- Result and Key Data:
- VGPox 1 and VGPox 2 sera exhibited strong neutralization (87.8% and 93.4%, respectively).
- All three vaccines conferred 100% survival in the rapid protection model.
Figure 5.The neutralizing activity of sera collected on day 7 post-vaccination was measured by VACV PRNT. Sera from the DPBS group were considered as 0% neutralization. n = 4 biologically independent mice.
Figure 6.On day 8 post-vaccination, mice from each group were intranasally challenged with VACV-WR (1 × 106 PFU/ mouse in 20 µl), and their body weight was recorded daily to assess disease severity. n = 5 biologically independent mice.
- Significance of the Result: Demonstrates the potential for mRNA vaccines to provide fast and effective protection.
- Key Innovation: Single-dose regimen underscores the flexibility and efficacy of mRNA-based vaccines.
Conclusion
The study concluded that mRNA vaccines encoding fusion proteins of MPXV demonstrate superior immunogenicity and protective efficacy against VACV in mice. The rapid immune response and long-term protection observed suggest that these vaccines could be vital in countering orthopoxvirus outbreaks. However, limitations include the focus on VACV rather than direct studies with MPXV. Future research should explore comparative efficacy in non-human primates and further investigate the immunological mechanisms at play, potentially paving the way for innovative vaccination strategies against mpox and similar viruses.
Reference:
Hou, Fujun, et al. “mRNA vaccines encoding fusion proteins of monkeypox virus antigens protect mice from vaccinia virus challenge.” Nature communications 14.1 (2023): 5925.
