Editor:Nina
Key Preview
Research Question
- Does a novel replicating RNA (repRNA) vaccine elicit robust cellular immune responses, particularly from CD4+ and CD8+ T cells, to offer protection against Mycobacterium tuberculosis (M.tb) compared to protein-based vaccines?
Research Design and Strategy
- Animal Model: The study used C57BL/6 mice as a preclinical model to evaluate vaccine efficacy.
- Vaccine Platforms: Two vaccine platforms were tested: a protein subunit vaccine combined with a synthetic TLR4 agonist adjuvant and a novel RNA vaccine delivered using nanostructured lipid carriers.
- Vaccination Strategies: Homologous (same vaccine for prime and boost) and heterologous (RNA-prime, protein-boost) strategies were employed.
- Outcome Measures: Immune responses were correlated with bacterial burden following M.tb challenge.
Method
- Immunization Regimens: Single prophylactic and prime-boost strategies were employed.
- Immune Response Assessment: Flow cytometry was used to evaluate cellular and humoral immune responses, including cytokine production and T-cell proliferation.
- Bacterial Burden Evaluation: Pulmonary bacterial load was assessed following aerosolized M.tb challenges.
Key Results
- Both vaccine platforms significantly reduced pulmonary bacterial burden.
- The heterologous RNA-prime, protein-boost strategy yielded the greatest reduction in bacterial load.
- RNA-based vaccines effectively elicited CD8+ T-cell responses, critical for combatting intracellular pathogens like M.tb.
Significance of the Research
- This study provides one of the first comprehensive evaluations of an RNA-based vaccine for tuberculosis in a preclinical model.
- Highlights the potential of repRNA technologies in eliciting robust immune responses.
- Sets a foundation for further development of TB vaccines to address a global health crisis.
Introduction
The emergence of tuberculosis as a leading cause of death from infectious diseases necessitates a renewed focus on vaccine development. With an increase in TB cases and deaths reported in recent years, especially exacerbated by the COVID-19 pandemic, innovative solutions to combat this disease are critical. Traditional vaccines like BCG have limited efficacy, particularly in adults, highlighting the need for novel approaches that can provide better protection against M.tb. The current research builds on prior studies indicating that CD8+ T-cell responses play a crucial role in controlling TB infection, thus prompting an exploration of RNA vaccine technologies that could enhance these responses. The primary objective of this research is to assess the efficacy of a new RNA-based vaccine platform, focusing on its ability to induce protective immunity against M.tb.
Research Team and Objective
The research team, comprised of Sasha E. Larsen, Jesse H. Erasmus, Valerie A. Reese, Tiffany Pecor, Jacob Archer, Amit Kandahar, Fan-Chi Hsu, Katrina Nicholes, Steven G. Reed, Susan L. Baldwin, and Rhea N. Coler, conducted this study from the Seattle Children’s Research Institute and HDT BioCorp. Published in the journal *Vaccines*, the paper outlines the significance of exploring RNA-based platforms as potential game-changers in the fight against TB. The objective of the study was to evaluate the immunogenicity and protective efficacy of a novel repRNA vaccine against M.tb, with an eye toward improving current vaccine strategies.
Key Experimental Procedures
Vaccine Preparation
- Key Steps: ID91, a fusion protein composed of four M.tb antigens, was formulated as a protein-adjuvant vaccine and as an RNA-encoded vaccine using lipid nanoparticles.
- Results and Key Data: The RNA vaccine platform demonstrated efficient antigen expression and stability.
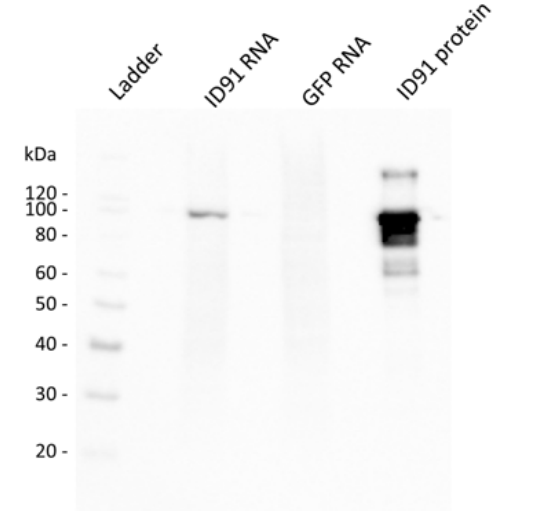
Figure1.Western blot of ID91 antigen expression from transfected BHK cell lysate in vitro after 24 h. From left to right: Ladder, Cell lysate from BHK transfected with ID91 RNA, GFP RNA, ID91 protein.
- Significance of the Result: Showcased the feasibility of using RNA technologies for M.tb antigen delivery.
- Key Innovation: Incorporation of nanostructured lipid carriers to enhance RNA vaccine delivery and immune activation.
Mouse Immunization
- Key Steps: Female C57BL/6 mice were immunized with either the protein-adjuvant or RNA vaccine. Prime-boost regimens were tested using homologous or heterologous strategies.
- Results and Key Data: Both vaccine platforms elicited significant immune responses, with the heterologous RNA-prime, protein-boost strategy producing the most robust T-cell responses.
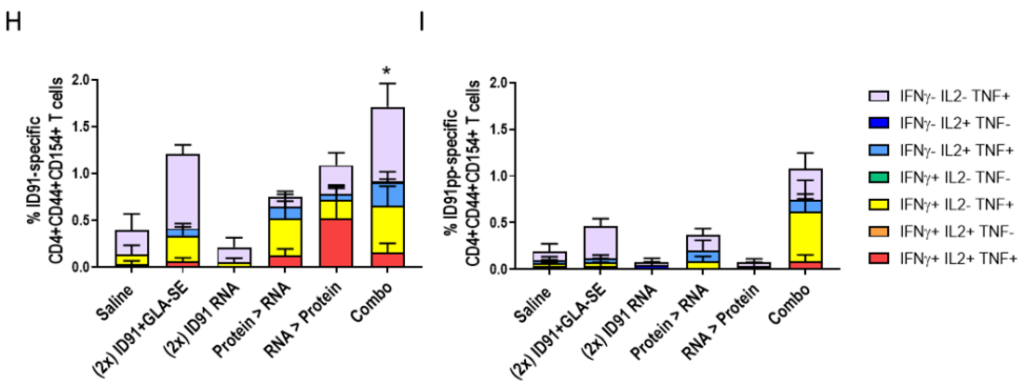
Figure 2. Single-cell suspensions isolated from the lungs of animals 3 weeks post-ULDA challenge were stimulated with ID91 protein or ID91 peptide pool and evaluated for CD4+ CD44+ CD154+ and CD8+ CD44+ T cell expression of IFNγ, IL-2 and TNF by flow cytometry.
- Significance of the Result: Demonstrated the superior efficacy of combining RNA and protein-based vaccines.
- Key Innovation: Use of a heterologous strategy to maximize immune response magnitude and breadth.
Immune Response Evaluation
- Key Steps: Flow cytometry was used to measure CD4+ and CD8+ T-cell responses, cytokine production (e.g., IFN-γ, TNF-α), and T-cell proliferation following immunization.
- Results and Key Data: The RNA vaccine elicited enhanced CD8+ T-cell activation, particularly in the heterologous prime-boost group.
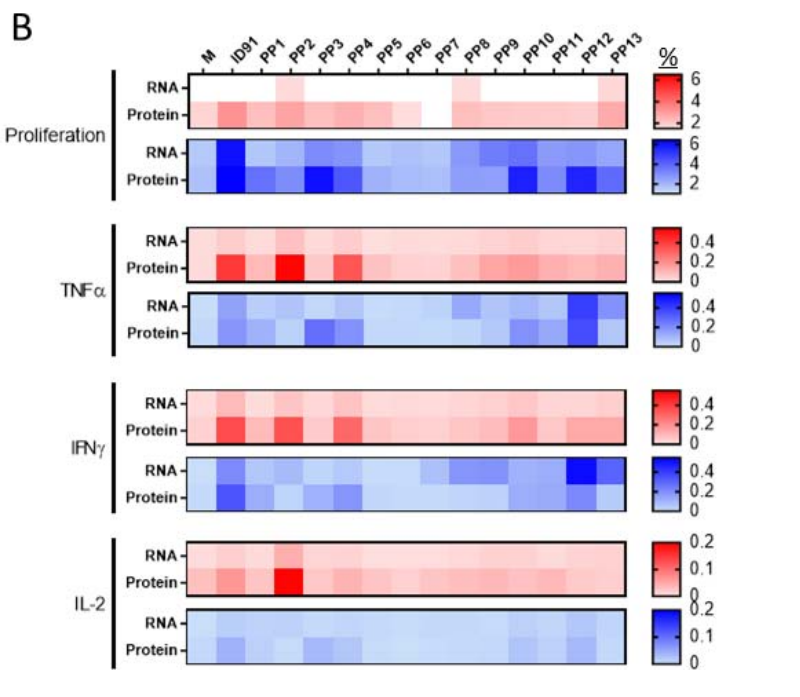
Figure 3. (B) Two weeks post-boost, splenocytes were stimulated with media (M) whole antigen (ID91) or 13 different peptide pools (PP) with overlapping 15 mers of ID91. Proliferation and cytokine expression were measured by flow cytometry after stimulation. Heat map depicts the percentage of CD4+ (red)- or CD8+ (blue)-responding T cells from each immunization depicted to the left (RNA or Protein). Data representative of a single experiment with n = 4 animals per group.
- Significance of the Result: Highlighted the role of CD8+ T cells in providing protection against M.tb.
- Key Innovation: Quantitative demonstration of RNA vaccines’ capacity to generate cellular immunity critical for TB control.
Pulmonary Bacterial Burden Assessment
- Key Steps: Mice were challenged with aerosolized M.tb and bacterial load in the lungs was quantified.
- Results and Key Data: Significant reductions in bacterial burden were observed in all vaccinated groups compared to controls, with the RNA-prime, protein-boost group showing the greatest reduction.
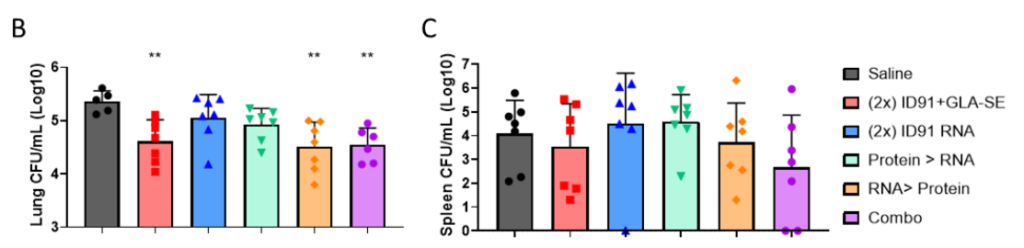
Figure 4. Bacterial burden assessed by colony-forming units (CFU) in (B) the lung and (C) spleen homogenates 3 weeks post-challenge. n = 6–7 mice per cohort, saline–grey,Vaccines 2023, 11, 130 11 of 17 homologous ID91 + GLA-SE–red, homologous ID91 repRNA +NLC–blue, protein prime/RNA boost– green, RNA prime/protein boost–orange, and combination–purple.
- Significance of the Result: Validated the protective efficacy of RNA-based vaccines against M.tb infection.
- Key Innovation: Demonstration of RNA vaccines’ potential to address challenges in TB vaccine development, particularly in eliciting effective cellular responses.
Cytokine Analysis
- Key Steps: Cytokine profiles of vaccinated mice were analyzed to assess the quality of immune responses.
- Results and Key Data: RNA-based vaccines promoted a Th1-biased immune response characterized by high levels of IFN-γ and IL-2.
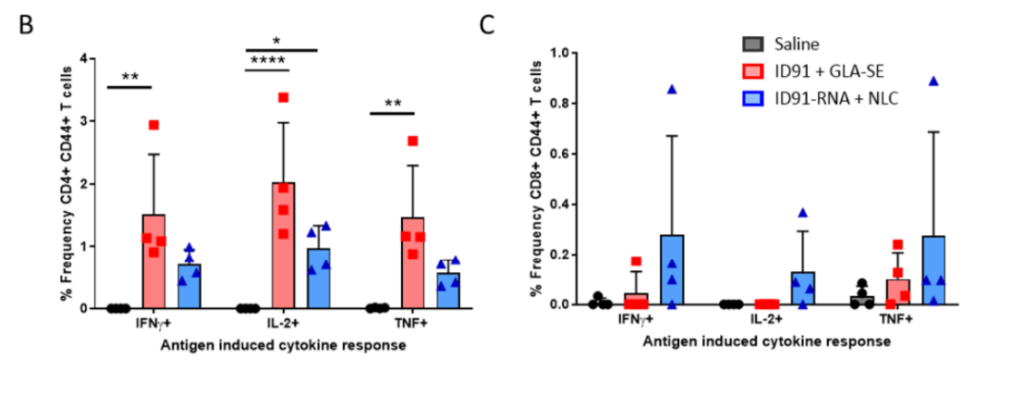
Figure5.Single immunization with repRNA vaccine is moderately immunogenic and affords prophylactic protection. (B) Percentage of CD4+CD44 cytokine-producing T cells as well as (C) percentage of CD8+CD44+cytokine-producing T cells after medium subtraction.
- Significance of the Result: Reinforced the role of Th1 responses in mediating protection against M.tb.
- Key Innovation: Use of cytokine profiling to provide mechanistic insights into vaccine-induced immunity.
Conclusion
The findings of this research underscore the potential of RNA-based vaccines in generating robust immune responses against M.tb, marking a significant advancement in TB vaccine development. The study illustrates that heterologous prime-boost strategies combining RNA and protein vaccines yield superior protective effects. Despite the promising results, the research acknowledges limitations, such as the need for further exploration of optimal dosing regimens and the evaluation of clinical isolates. Future research should focus on refining vaccine delivery methods and assessing long-term immune responses in diverse populations to maximize the efficacy of TB vaccines. The ongoing development of RNA vaccine technologies represents a critical step forward in addressing the global TB epidemic.
Reference:
Larsen, Sasha E., et al. “An RNA-based vaccine platform for use against Mycobacterium tuberculosis.” Vaccines 11.1 (2023): 130.
