Editor: Nina
Key Preview
Research Question
- Can spironolactone-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles enhance the treatment of Schistosoma mansoni infections by improving drug solubility, efficacy, and delivery while reducing the frequency of administration?
Research Design and Strategy
- The study was conducted to address the limitations of praziquantel (PZQ), the standard treatment for schistosomiasis, focusing on poor efficacy against immature stages and emerging drug resistance.
- Researchers formulated spironolactone (SPL)-loaded PLGA nanoparticles using nanoprecipitation and emulsion solvent evaporation methods to test their potential as an antischistosomal therapy.
- Experimental validation was performed using in vitro and in vivo models.
Method
- Nanoparticle Preparation: Spironolactone was encapsulated in PLGA using two methods: nanoprecipitation (with acetone) and emulsion solvent evaporation (with dichloromethane).
- Characterization: Nanoparticles were characterized for size, zeta potential, drug entrapment efficiency, and release kinetics. Key parameters included an average size of 238 nm, zeta potential of -19.66 mV, and encapsulation efficiency of 90.43%.
- In Vitro Testing: Release studies demonstrated sustained biphasic drug release governed by Fickian diffusion.
- In Vivo Testing: Murine models infected with S. mansoni were treated with SPL nanoparticles, free SPL, or PZQ. Parasite burdens, egg loads, and liver pathology were assessed.
Key Results
- SPL-loaded PLGA nanoparticles reduced liver and spleen indices, total worm counts, and egg loads more effectively than free SPL.
- Hepatic egg loads were reduced by 57.75%, and intestinal egg loads decreased by 54.17% in the nanoparticle treatment group.
- SPL nanoparticles caused extensive damage to schistosomal tegument, including disruption of suckers and exposure of subtegumental tissue.
- Improved liver pathology was observed, including reduced granuloma size and fibrosis.
Significance of the Research
- The study demonstrates the potential of nanoparticle-based drug delivery systems to enhance therapeutic outcomes in schistosomiasis treatment.
- SPL nanoparticles provide a promising alternative to praziquantel, addressing key limitations such as poor solubility and resistance.
Introduction: Addressing a Global Health Challenge
Schistosomiasis, caused by the Schistosoma parasite, remains a critical public health issue, particularly in developing countries. The disease infects approximately 240 million people globally, leading to considerable morbidity and mortality. While praziquantel has been the mainstay of treatment, it has several drawbacks, including limited effectiveness against immature forms of the parasite and the emergence of drug-resistant strains. This study represents a step towards innovation in treatment, utilizing nanoparticles to enhance drug delivery and efficacy. The necessity of this research lies in the urgent need for more effective therapies that can overcome the limitations of existing treatments, thereby improving health outcomes for infected populations.
Research Team and Objectives
The research was conducted by a team from Mansoura University, including Walaa Ebrahim Abd El Hady, Ghada Ahmed El-Emam, Nora E Saleh, Marwa M Hamouda, and Amira Motawea. Their findings were published in the International Journal of Nanomedicine. The primary objective of the study was to develop and assess the antischistosomal effects of spironolactone-loaded PLGA nanoparticles, focusing on their potential to serve as a safer and more effective treatment alternative to praziquantel.
Experimental Design and Key Findings
Key Experimental Procedures
- Nanoparticle Formulation and Characterization
Key Steps: SPL was encapsulated in PLGA using two methods—nanoprecipitation (acetone) and emulsion solvent evaporation (dichloromethane). Nanoparticles were freeze-dried and characterized.
Results: Optimized nanoparticles (F4) had a size of 238 nm, encapsulation efficiency of 90.43%, and zeta potential of -19.66 mV.
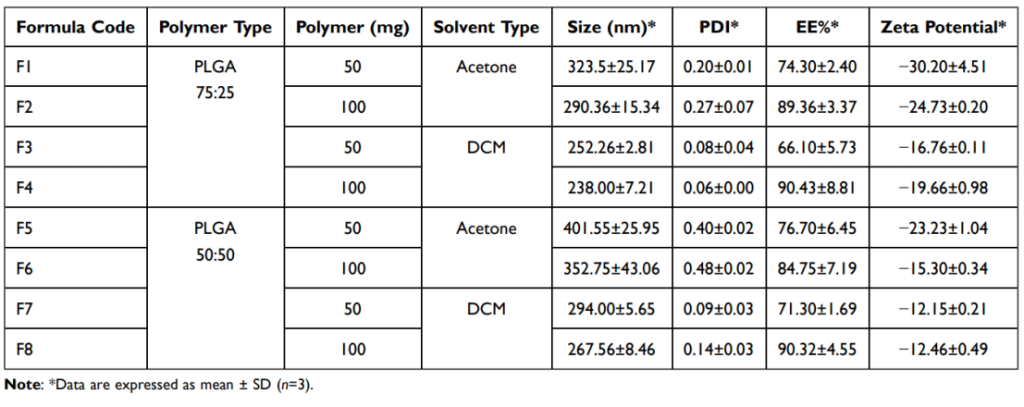
Table 1: Characterization of the Prepared Nanoparticles, Each Formula Containing 25 mg Drug
Significance: These parameters ensure stability, high drug loading, and sustained release.
2. In Vitro Drug Release Studies
Key Steps: Nanoparticles were subjected to dissolution studies in 0.1N HCl (pH 1.2) and phosphate buffer (pH 6.8) using the USP paddle method.
Results: SPL-loaded PLGA nanoparticles exhibited a biphasic release pattern, with up to 75% drug release over 24 hours.
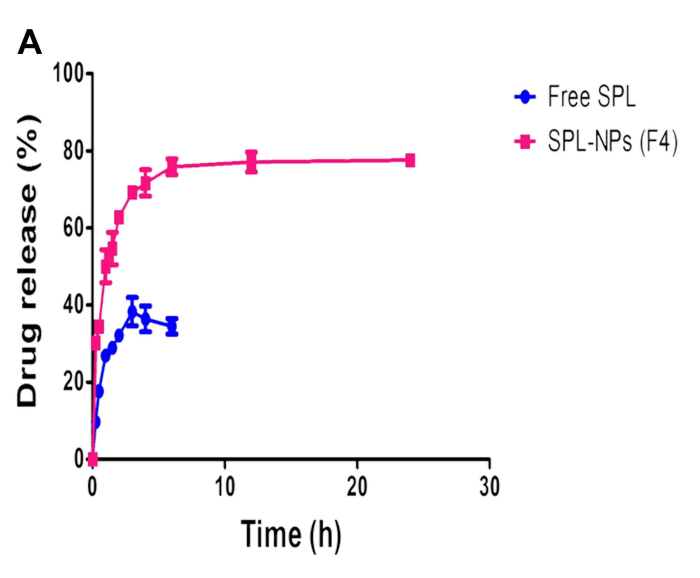
Figure 1 In vitro dissolution results in: (A) 0.1N HCl (pH 1.2) from the optimized SPL PLGA NPs (F4) compared to the free drug
Significance: The sustained release profile enhances therapeutic efficacy and reduces dosing frequency.
3. In Vivo Treatment and Parasitological Assessment
Key Steps: Swiss albino mice infected with S. mansoni were treated with SPL nanoparticles, free SPL, or PZQ at 6 weeks post-infection. Liver and spleen indices, worm burden, and egg loads were measured.
Results: SPL nanoparticles reduced liver and spleen indices by 22.28% and 39.32%, respectively.
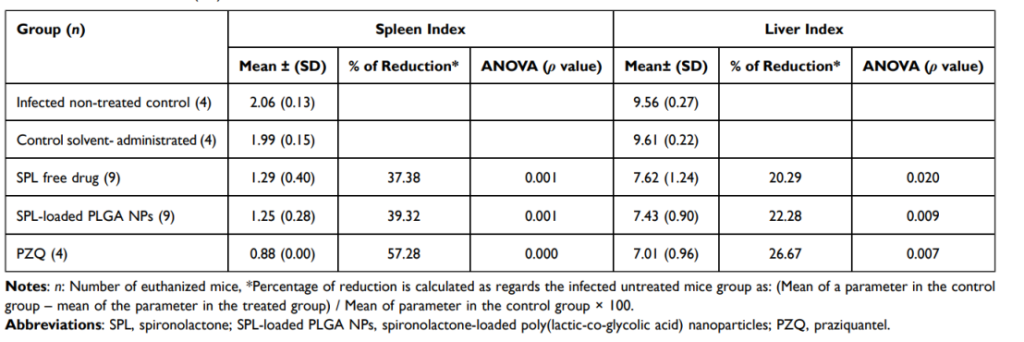
Table 2 Liver and Spleen Indices Following Treatment of Schistosoma mansoni (Egyptian Strain)-Infected Mice by SPL Free Drug, SPL-Loaded PLGA NPs (F4), and PZQ
Total worm burden was reduced by 61.54%, comparable to PZQ.
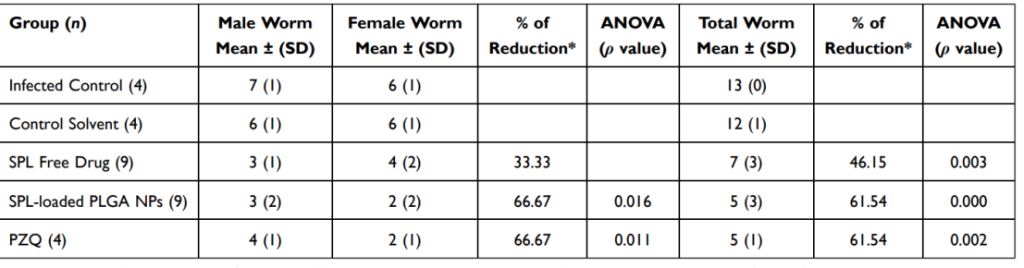
Table 3 Worm Burden Following Treatment of Schistosoma mansoni (Egyptian Strain)-Infected Mice by SPL Free Drug, SPL-Loaded PLGA NPs (F4), and PZQ
Hepatic and intestinal egg loads decreased by 57.75% and 54.17%, respectively.
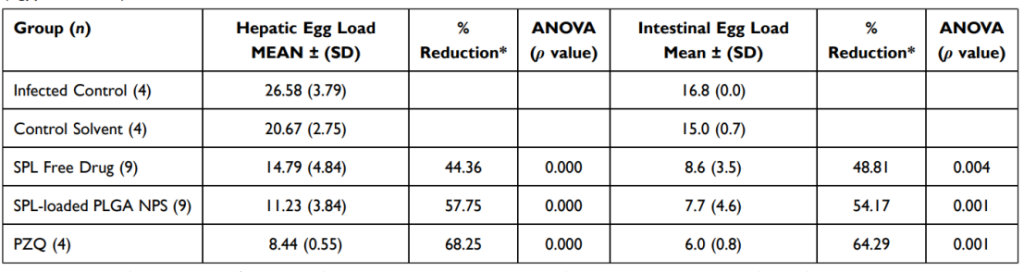
Table 4 Tissue Egg Load Following Treatment by SPL Free Drug, SPL-Loaded PLGA NPs (F4), and PZQ of Schistosoma mansoni (Egyptian Strain)-Infected Mice
Significance: SPL nanoparticles demonstrated superior efficacy over free SPL and matched PZQ in reducing parasitological burdens.
4. Scanning Electron Microscopy
Key Steps: Worms recovered post-treatment were examined under SEM to assess tegumental damage.
Results: SPL nanoparticles caused extensive tegumental erosion, fissures, and subtegumental exposure in male and female worms.
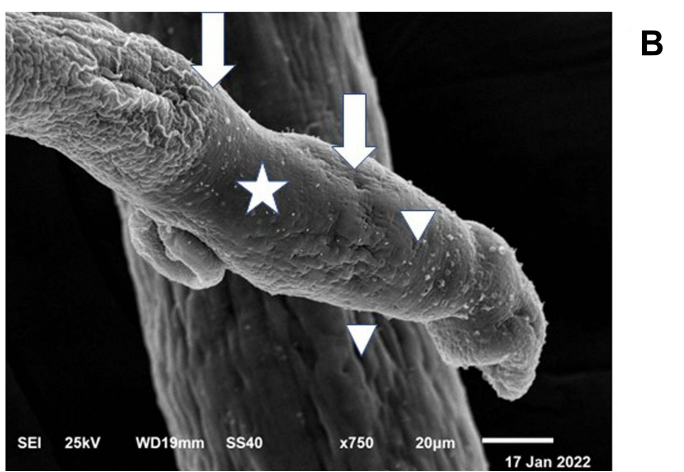
Figure 2 (B) The dorsal surface of the female worm also showed extensive erosions and fissures (star) making holes with exposing of subtegumental tissue (arrow), and also peeling of tegument and disruption of folds integrity was seen (arrowhead)
Significance: Tegumental disruption impairs parasite survival and reproduction, explaining the reduced egg loads and worm counts.
5. Histopathological Analysis
Key Steps: Liver tissue from treated mice was examined for granuloma formation and fibrosis.
Results: SPL nanoparticles significantly reduced granuloma size and inflammation compared to untreated controls.
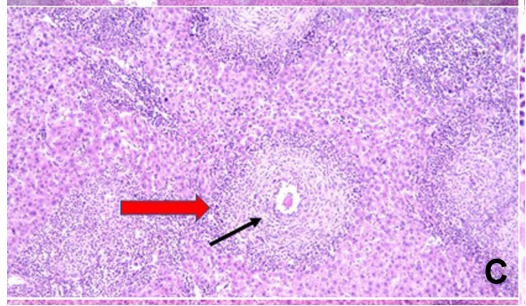
Figure 3 (C) showing evident decrease in the number of epithelioid granulomas having an ovum in the center of few of them (black arrow) (H=E; magnification×100)
Significance: Improved liver pathology suggests reduced disease severity and inflammation.
Conclusion: Implications and Future Directions
The study concludes that spironolactone-loaded PLGA nanoparticles represent a promising new avenue for treating schistosomiasis, demonstrating substantial improvements in drug efficacy and reduced side effects compared to traditional therapies. While the findings are encouraging, the study acknowledges limitations, such as the need for extensive clinical evaluations and the exploration of drug resistance mechanisms. Future research should focus on in-depth studies regarding the long-term efficacy of the nanoparticles and their potential integration into existing treatment protocols for schistosomiasis, paving the way for enhanced therapeutic strategies against this neglected tropical disease.
Reference:
Abd El Hady, Walaa Ebrahim, et al. “The idiosyncratic efficacy of spironolactone-loaded PLGA nanoparticles against murine intestinal schistosomiasis.” International Journal of Nanomedicine (2023): 987-1005.
