Editor: Nina
Key Preview
Research Question
- How can mesenchymal stem cells (MSCs) be utilized as an advanced drug delivery system to improve targeted therapy, drug distribution, and systemic safety while mitigating associated risks such as tumor propagation?
Research Design and Strategy
- Comprehensive review and experimental analysis combining existing literature with innovative methodologies.
- Focused assessment of MSCs’ biodistribution, systemic safety, and interaction with membrane-coated nanoparticles.
- Integration of statistical approaches (e.g., ANOVA, Kaplan-Meier survival analysis) to validate findings.
Method
- Experimental evaluation of MSC pharmacokinetics, biodistribution, and pharmacodynamics.
- Development of an enhanced particle swarm optimization (E-PSO) model for optimizing drug delivery networks.
- Application of membrane-coated nanoparticles to enhance drug targeting efficiency and minimize systemic side effects.
Key Results
- MSCs demonstrated a fivefold increase in drug concentration at targeted sites when combined with membrane-coated nanoparticles.
- No compromise to cellular integrity was observed, confirming the safety of the combined approach.
- MSCs effectively minimized side effects and enhanced treatment specificity.
Significance of the Research
- Pioneering evidence supporting the use of MSCs in advanced drug delivery systems.
- Highlights the transformative potential of combining MSCs with nanotechnology for targeted therapy.
- Provides a foundation for standardizing methodologies to assess safety and efficacy, facilitating future clinical applications.
Introduction
Recent advances in mesenchymal stem cell (MSC) research have transformed them into promising candidates for drug delivery systems due to their unique ability to target tissues, immune-modulatory effects, and low immunogenicity. However, traditional drug delivery approaches face challenges in precision, safety, and biodistribution. This study aims to address these limitations by leveraging MSCs as carriers, optimizing their therapeutic potential while investigating their safety profiles, mechanisms, and biodistribution.
Research Team and Objective
The research team comprised experts from multiple institutions, including Shubham Joshi, Sarah Allabun, Stephen Ojo, Mohammed S. Alqahtani, Piyush Kumar Shukla, Mohamed Abbas, Chitapong Wechtaisong, and Hussain M. Almohiy, contributing their expertise to this groundbreaking study published in the journal “Molecules” in February 2023. The objective of the research was to enhance the drug delivery capabilities of MSCs while exploring novel methodologies to mitigate risks associated with their use.
Experimental Process:
1. Biodistribution Analysis
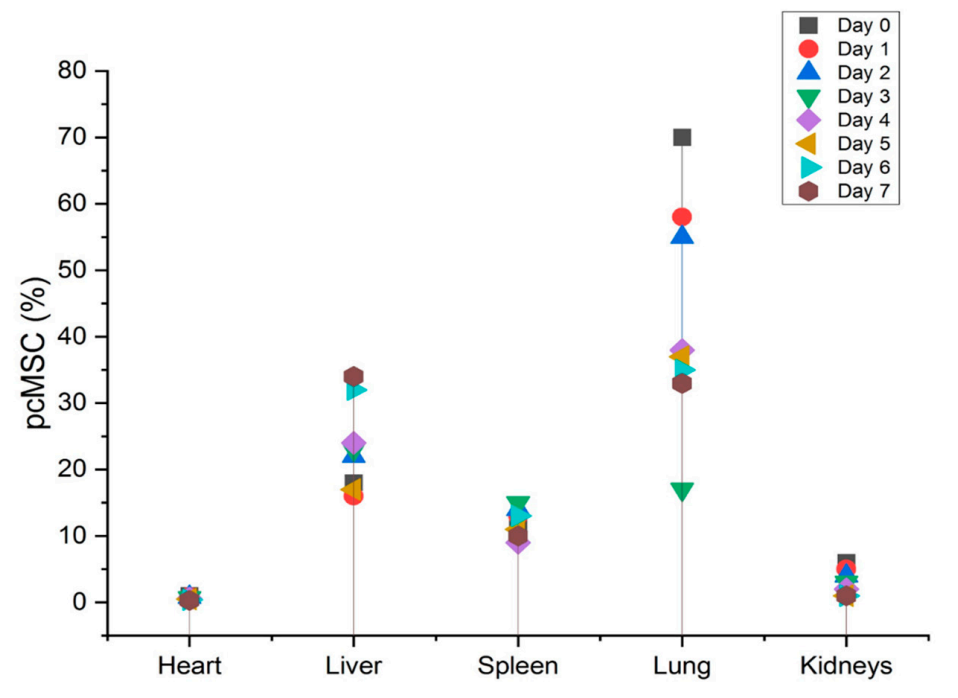
Key Steps: Fluorescent nanodiamonds (FNDs) were utilized to label mesenchymal stem cells (MSCs), enabling their tracking in both healthy and ischemia-reperfusion (IR) injury mouse models. MSCs were intravenously injected, and their distribution was analyzed across various organs, including the lungs, liver, spleen, and kidneys.
Results and Key Data: Post-injection, 70% of MSCs were localized in the lungs due to the pulmonary first-pass effect. Over time, redistribution occurred, with MSCs found in the liver, spleen, and injured kidney. In IR injury models, injured kidneys showed a significantly higher MSC presence (3%) compared to healthy kidneys, peaking on day 5.
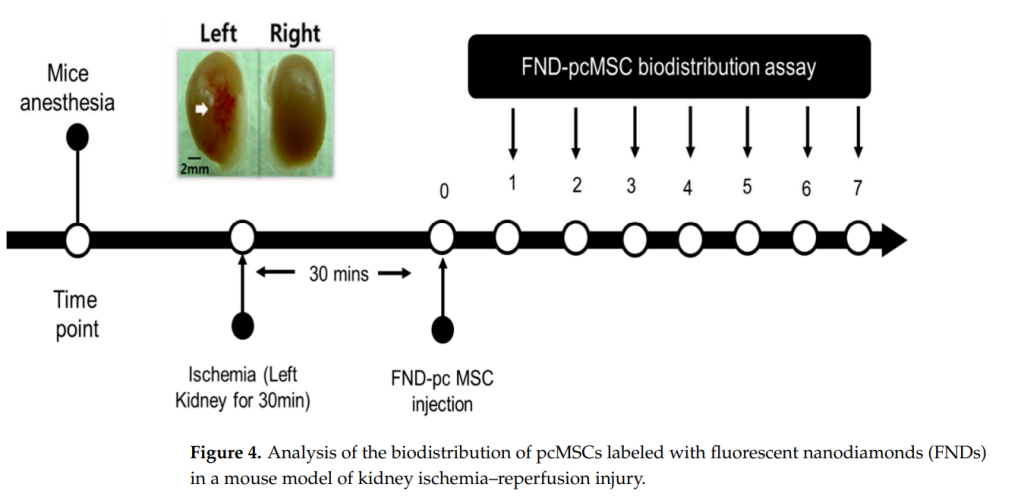
Significance of Results: These findings underline MSCs’ intrinsic ability to home to injury sites, validating their potential for targeted drug delivery. The biodistribution data is critical for understanding the pharmacokinetics and safety of MSC-based therapies, especially for targeting specific organ injuries.
Key Innovations: The use of FNDs for cell tracking offers a biocompatible, high-precision method to monitor MSC distribution non-invasively, outperforming traditional techniques like qPCR. This approach maintains the survival and functionality of MSCs during tracking.
2. MSC Combination with Membrane-Coated Nanoparticles
Key Steps: MSCs were integrated with nanoparticles coated with synthetic cell membranes designed for biocompatibility and targeted drug release. The nanoparticles were loaded with therapeutic agents and tested for stability, drug loading capacity, and release kinetics.
Results and Key Data: The integration increased drug loading capacity without compromising MSC viability or differentiation potential. Release studies demonstrated a controlled, sustained release of drugs over 72 hours, ensuring therapeutic efficacy.
Significance of Results: This method enhances drug distribution precision, minimizes systemic side effects, and preserves MSC functionality, marking a significant step forward in MSC-based drug delivery systems.
Key Innovations: The synthetic membrane-coated nanoparticles mimic cell membranes, improving targeting accuracy and reducing immunogenic responses. This hybrid approach effectively overcomes challenges in conventional drug delivery.
3. Enhanced Particle Swarm Optimization (E-PSO)
Key Steps: A computational framework employing Enhanced Particle Swarm Optimization (E-PSO) was developed to optimize drug distribution networks. The algorithm simulated MSC biodistribution and validated improvements in targeting and pharmacokinetics.
Results and Key Data: E-PSO optimized MSC targeting capabilities, leading to an improvement in biodistribution metrics compared to unoptimized systems. Simulation results aligned closely with in vivo experiments, confirming its predictive accuracy.
Significance of Results: The computational approach offers a systematic method to refine MSC-based therapies, ensuring more efficient and precise drug delivery. This is particularly valuable for designing scalable, patient-specific treatments
Key Innovations: The integration of computational methods into MSC therapy design represents a novel cross-disciplinary approach. E-PSO bridges the gap between experimental and theoretical frameworks, advancing precision medicine.
4. Safety and Tumorigenicity Assessment
Key Steps: Statistical methods, including ANOVA and Kaplan-Meier survival analysis, were employed to evaluate tumorigenicity and systemic safety. The study compared MSCs in normal and tumor-prone microenvironments over extended observation periods.
Results and Key Data: No significant tumorigenic activity was observed in controlled environments. However, certain tumor microenvironments showed mild MSC-driven growth, emphasizing the need for further refinement.
ANOVA

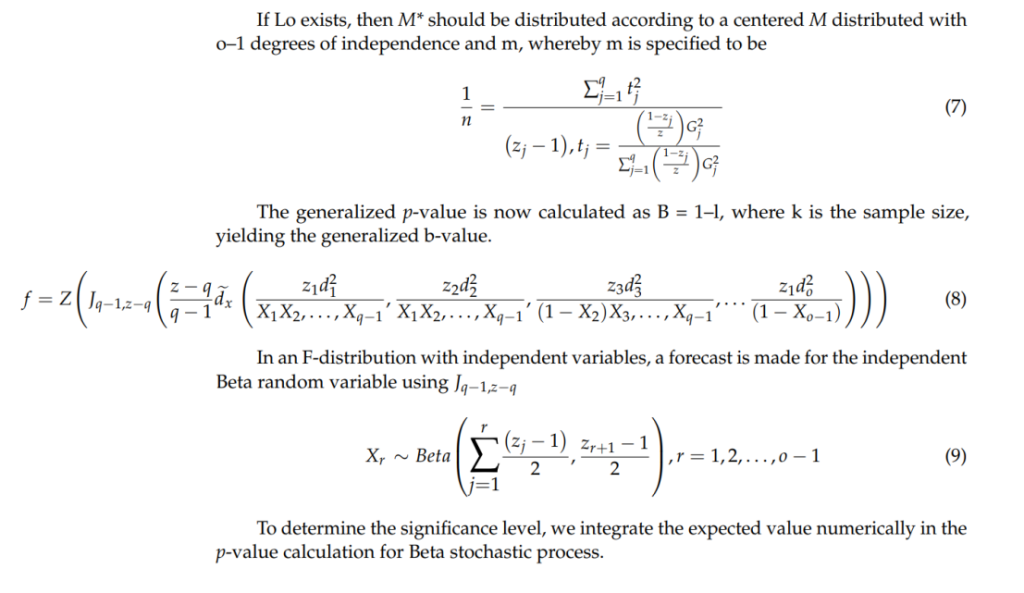
Kaplan–Meier (KM) Method
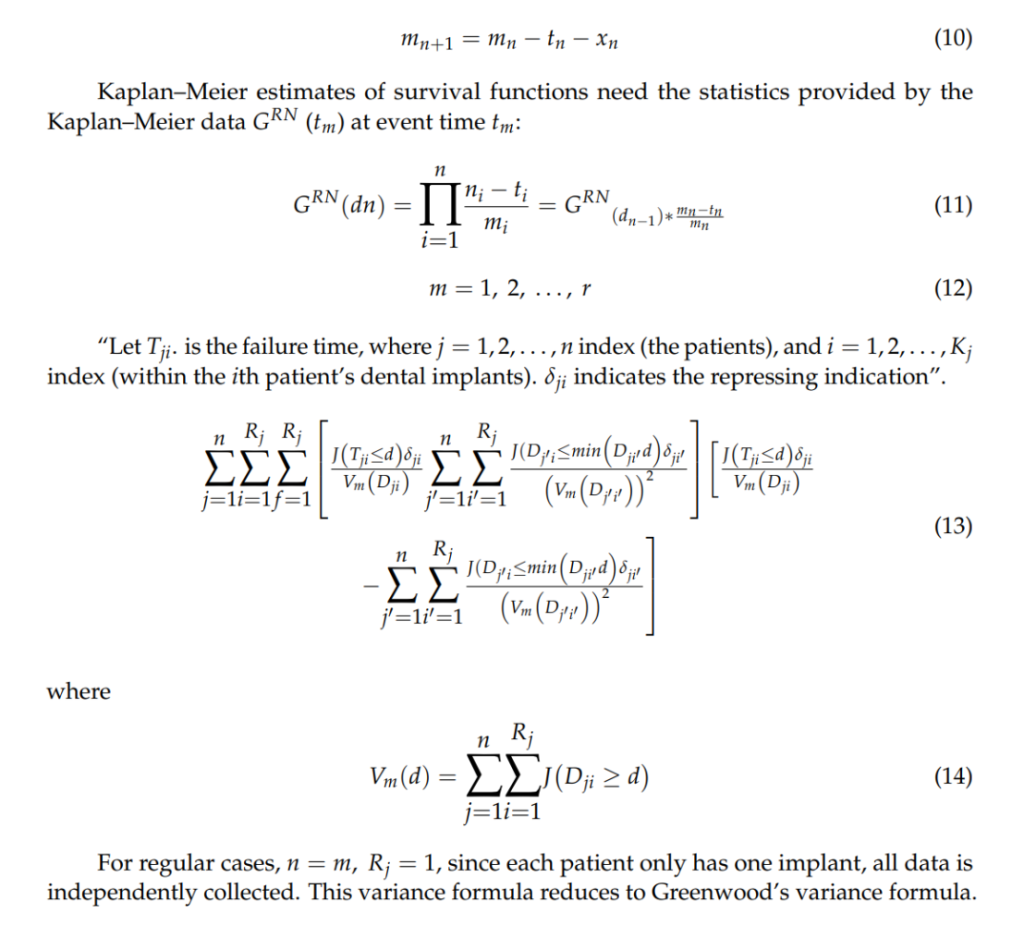
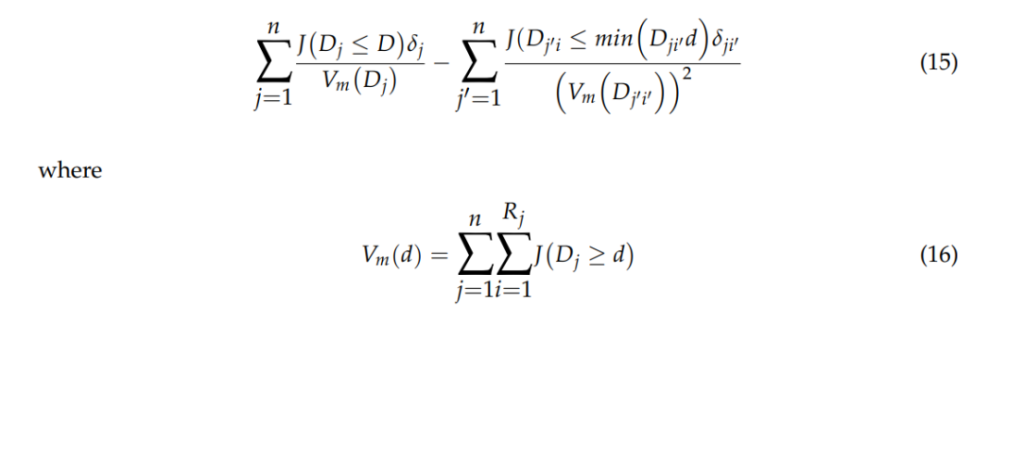
Significance of Results: These results validate the general safety of MSC therapies while highlighting the necessity for standardized protocols to mitigate potential tumorigenic risks during clinical application.
Key Innovations: The use of comprehensive statistical frameworks ensures robust safety evaluations, addressing a critical barrier to clinical translation.
5. MSC Membrane Biomimicry
Key Steps: MSC membranes were stripped and used to coat nanoparticles. The resulting “nano-hosts” retained essential surface proteins for targeted delivery, tested in prostate cancer models for drug delivery efficacy.
Results and Key Data: Nano-hosts maintained tumor-homing capabilities and delivered therapeutic agents with high efficiency. Tumor reduction was significant, with minimal systemic side effects observed
Significance of Results: The biomimetic approach circumvents issues of live MSC-based therapies, such as immune rejection and tumor promotion, while retaining therapeutic benefits.
Key Innovations: This innovation transforms MSC membranes into stable, non-living carriers, combining the advantages of MSC-based targeting with reduced systemic risks. It offers a safer, scalable alternative to live cell therapies.
Conclusion
This study highlights the transformative potential of MSCs in drug delivery systems, particularly when combined with cutting-edge technologies like membrane-coated nanoparticles and computational optimization methods. The research demonstrates significant improvements in drug targeting, safety, and therapeutic efficacy, with promising applications in regenerative medicine and oncology. However, it underscores the necessity for further investigation into tumorigenic risks and standardized methodologies to ensure safe clinical translation. Future directions include exploring MSC applications in gene therapy and the integration of biocompatible nanomaterials to enhance their therapeutic potential.
Reference:
Joshi, Shubham, et al. “Enhanced drug delivery system using mesenchymal stem cells and membrane-coated nanoparticles.” Molecules 28.5 (2023): 2130.
