Editor: Nina
Key Preview
1.Research Question
Can retinoic acid-modified nanoparticles improve the therapeutic efficacy of galangin for the treatment of hepatic fibrosis (HF)?
2.Research Design and Strategy
The research focused on creating a novel drug delivery system using nanoparticles modified with retinoic acid to target hepatic stellate cells (HSC).
A systematic experimental approach was adopted to assess the properties of the nanoparticles, including their size, encapsulation efficiency, drug release profiles, and their targeting ability for HSC.
3.Method
The nanoparticles were synthesized using Eudragit® RS100 and modified with retinoic acid.
The physicochemical properties were characterized, and pharmacokinetic studies were conducted to evaluate drug release and bioavailability.
A series of in vivo studies were performed to assess the therapeutic impact on HF, specifically focusing on fibrosis markers and HSC targeting.
4. Key Results
The nanoparticles demonstrated an average size of approximately 70 nm and a high encapsulation efficiency (>95%).
Galangin delivered through these nanoparticles exhibited controlled release and maintained higher plasma concentrations over time.
The treatment resulted in significant reductions in markers of hepatic fibrosis, indicating a marked anti-fibrotic effect.
5. Significance of the Research
The study presents a novel strategy to enhance the delivery and therapeutic effects of galangin, overcoming its hydrophobic limitations.
The use of retinoic acid-modified nanoparticles opens new avenues for more effective treatments for hepatic fibrosis, a condition with limited treatment options.
The approach holds promise for improving the clinical management of liver diseases, particularly those related to fibrosis.
Introduction
Hepatic fibrosis (HF) is a progressive liver condition caused by chronic liver injury that can eventually lead to cirrhosis and liver cancer. One of the key drivers of HF is the activation of hepatic stellate cells (HSC), which contribute to excessive extracellular matrix deposition in the liver. Current treatments for HF are limited, emphasizing the need for targeted therapies.
This study introduces a novel drug delivery strategy utilizing retinoic acid-modified nanoparticles aimed at enhancing the bioavailability and therapeutic efficacy of galangin. Galangin is a flavonoid with promising anti-fibrotic properties, but its hydrophobic nature limits its clinical effectiveness. By targeting HSC, which play a pivotal role in fibrosis progression, the research aims to develop a more efficient drug delivery system.
The main research question addressed in this study is whether retinoic acid-modified nanoparticles can improve the therapeutic efficacy of galangin in treating HF.
Research Team and Objective
The research team, consisting of experts from various institutions in China, sought to develop a targeted drug delivery system using nanoparticles modified with retinoic acid. These nanoparticles, made of Eudragit® RS100, were designed to overcome the hydrophobic limitations of galangin and enhance its bioavailability.
The objective was to evaluate whether these nanoparticles could effectively target HSCs and improve the therapeutic effects of galangin in vivo, providing a promising new approach to treat HF.
Experimental Process
- Synthesis and Characterization of Nanoparticles
Step: The nanoparticles were synthesized using Eudragit® RS100 and modified with retinoic acid to enhance their targeting ability for HSC.
Result: The nanoparticles had an average size of 70 nm and a high encapsulation efficiency of over 95%.

Significance: The small size of the nanoparticles allows efficient cellular uptake, while the high encapsulation efficiency ensures effective delivery of galangin.
Innovation: The use of retinoic acid for nanoparticle modification enhanced the specificity of drug delivery to HSCs, a novel approach in targeting liver fibrosis.
2. Drug Release Profile
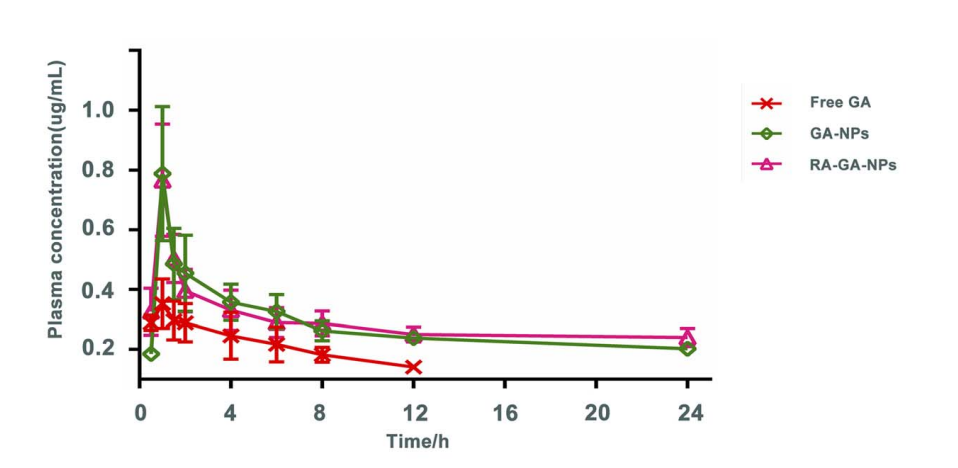
Step: The release profile of galangin from the nanoparticles was evaluated over time using in vitro release assays.
Result: The nanoparticles demonstrated controlled release of galangin, maintaining steady plasma concentrations over a prolonged period.
Significance: Controlled drug release is essential for maintaining therapeutic drug levels in the liver, improving treatment efficacy.
Innovation: The controlled release of galangin from these nanoparticles enhances its bioavailability and extends its therapeutic window, overcoming the limitations of free galangin.
3. Pharmacokinetic Studies
Step: Pharmacokinetic analysis was performed to compare the plasma concentration of galangin delivered via nanoparticles with that of free galangin.
Result: Nanoparticle-mediated delivery of galangin resulted in significantly higher plasma concentrations over time compared to free galangin.
Significance: Higher plasma concentrations of galangin indicate better drug retention and extended therapeutic effects, crucial for treating chronic conditions like HF.
Innovation: This long-lasting presence of galangin in the bloodstream offers a clear advantage over conventional delivery methods that result in rapid drug clearance.
4. In Vivo Evaluation of Anti-Fibrotic Effects
Step: In vivo studies were conducted on animal models of hepatic fibrosis to evaluate the effect of galangin-loaded nanoparticles on fibrosis markers.
Result: Treatment with retinoic acid-modified nanoparticles significantly reduced fibrosis markers in liver tissue, suggesting a potent anti-fibrotic effect.
Significance: The reduction in fibrosis markers indicates that the targeted delivery of galangin to HSCs effectively mitigates liver fibrosis.
Innovation: This represents a key advancement in liver fibrosis therapy, as it provides a more specific and efficient method for targeting HSCs compared to traditional systemic treatments.
5. Targeting Hepatic Stellate Cells (HSCs)
Step: The ability of the nanoparticles to target and accumulate in HSCs was assessed using fluorescence microscopy.
Result: The retinoic acid-modified nanoparticles showed enhanced targeting of HSCs compared to non-modified nanoparticles.
Significance: The targeting of HSCs is crucial for addressing the root cause of hepatic fibrosis, as these cells are central to the fibrotic process.
Innovation: Retinoic acid’s role in enhancing the specificity of nanoparticle targeting marks a novel contribution to precision medicine in liver disease treatment.
Conclusion
This study represents a significant advancement in the targeted treatment of hepatic fibrosis. The retinoic acid-modified galangin nanoparticles effectively enhance the bioavailability and therapeutic efficacy of galangin, overcoming its hydrophobic limitations and offering a promising strategy for liver fibrosis therapy. The ability of these nanoparticles to target HSCs with high precision marks a critical innovation in drug delivery systems for liver diseases.
However, the study acknowledges the need for further research, particularly regarding the long-term safety and clinical applicability of these nanoparticles. Future studies may focus on optimizing the nanoparticle system further and exploring the molecular mechanisms underlying their therapeutic effects on HSCs. Overall, this approach opens new avenues for the development of more effective treatments for liver fibrosis, a condition with currently limited therapeutic options.
Reference:
Xiong, Yuanguo, et al. “Galangin delivered by retinoic acid-modified nanoparticles targeted hepatic stellate cells for the treatment of hepatic fibrosis.” RSC advances 13.16 (2023): 10987-11001.
