Editor: Nina
Key Preview
1. Research Question
What is the therapeutic potential of Lip-BBR in reversing liver damage and improving metabolic functions in T2DM?
2. Research Design and Strategy
Multidisciplinary Approach: The study is conducted by a team of researchers from various institutions, combining expertise in metabolic disorders, pharmacology, and biochemistry.
Animal Model: The research utilizes diabetic Sprague-Dawley rats, allowing for controlled experimental conditions and accurate simulation of T2DM.
Comparison Groups: The study compares Lip-BBR with established therapies, such as vildagliptin, to assess its relative effectiveness in reversing liver damage and improving metabolic outcomes.
3. Method
Experimental Design: Diabetic rats were divided into multiple groups, including a control group, a Lip-BBR treatment group, a vildagliptin treatment group, and a combination therapy group.
Treatment Protocol: Lip-BBR (10 mg/kg body weight) was administered to the rats for 14 weeks, with the primary outcome measures being liver tissue regeneration, metabolic regulation, and insulin sensitivity.
4. Key Results
Liver Histology: Lip-BBR treatment significantly restored liver microarchitecture and reduced liver steatosis (fat accumulation in the liver).
Autophagy Activation: The treatment enhanced autophagy via the AMPK/mTOR signaling pathway, which is crucial for cellular energy regulation.
Metabolic Outcomes: Lip-BBR significantly reduced fasting blood glucose and improved insulin sensitivity, with the combination of Lip-BBR and vildagliptin showing a synergistic effect in regulating glucose metabolism.
5. Significance of the Research
Novel Therapeutic Approach: This study presents Lip-BBR as a promising treatment for T2DM-related liver injury, which has limited effective therapeutic options.
Bioavailability Enhancement: The formulation of berberine within liposomes addresses prior challenges related to the compound’s solubility and bioavailability, making it a more effective treatment option.
Impact on Diabetes Management: The findings suggest that Lip-BBR could play a key role in improving the management of diabetes and its complications by targeting both metabolic dysfunctions and liver damage.
Research Team and Objective
The study, led by a multidisciplinary team of researchers from various institutions, seeks to explore the efficacy of Lip-BBR in reversing liver damage caused by T2DM. The research questions primarily focus on understanding the therapeutic potential of Lip-BBR in restoring liver function, enhancing insulin sensitivity, and regulating lipid metabolism. Utilizing a well-defined experimental design, the researchers conducted a series of tests on diabetic rats, comparing the effects of Lip-BBR treatment to standard therapies, including vildagliptin.
Experimental Process
The experimental process was carefully designed to assess the effects of Lip-BBR on liver function and metabolic regulation in a diabetic animal model. Below are the key experimental procedures, results, and their significance:
1. Induction of Diabetes in Rats
Procedure: Type 2 diabetes was induced in male Sprague-Dawley rats using a high-fat diet followed by the administration of low-dose streptozotocin (STZ).
Result: This resulted in a diabetic state characterized by hyperglycemia, insulin resistance, and liver dysfunction, mimicking the pathophysiology of T2DM.
Significance: This animal model provided a relevant platform to evaluate the effects of therapeutic interventions, particularly Lip-BBR, on liver injury and metabolic disturbances in T2DM.
2. Group Assignment and Treatment Protocol
Procedure: The rats were divided into the following treatment groups:
Control group (no treatment)
Lip-BBR group (10 mg/kg body weight for 14 weeks)
Vildagliptin group (as a comparison treatment)
Combination group (Lip-BBR + vildagliptin)
Result: These groups allowed for a comparative analysis of the effectiveness of Lip-BBR against standard treatments.
Significance: The experimental design ensured that the impact of Lip-BBR could be compared to existing therapies, validating its therapeutic potential.
3. Liver Histopathology Analysis
Procedure: After the 14-week treatment period, liver tissues were collected and subjected to histological analysis to assess microarchitecture and the extent of steatosis (fat accumulation in the liver).
Result: Lip-BBR treatment resulted in significant restoration of normal liver architecture and a marked reduction in hepatic steatosis compared to the diabetic control group.
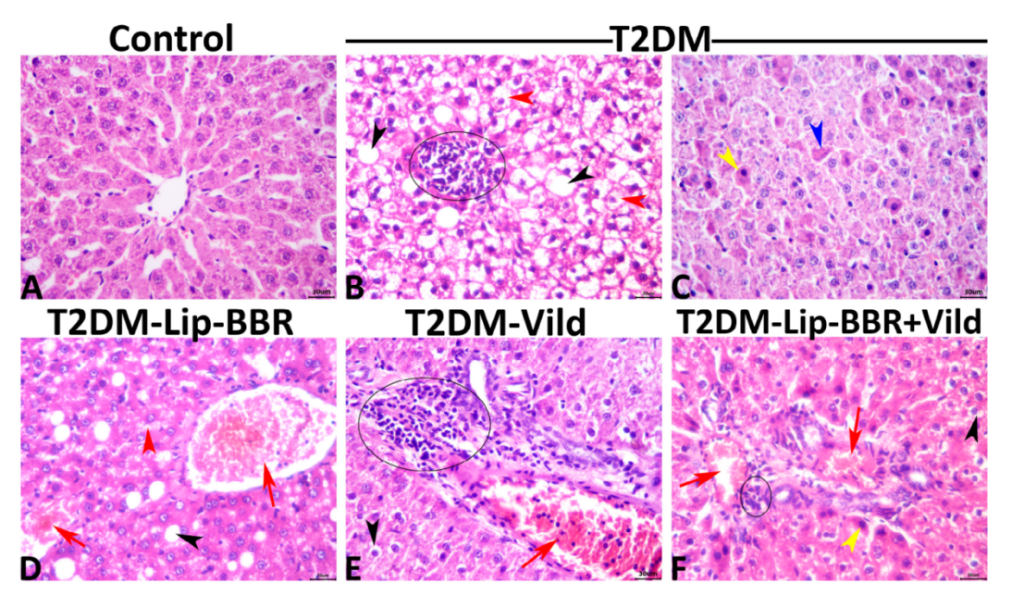
Significance: This finding suggests that Lip-BBR has the potential to protect the liver from the structural damage caused by T2DM, highlighting its hepatoprotective effects.
4. Assessment of Autophagy Activation
Procedure: The activation of autophagy was assessed by measuring the expression levels of key markers (e.g., LC3-II and p62) and the AMPK/mTOR signaling pathway.
Result: Lip-BBR treatment significantly upregulated autophagy markers, demonstrating enhanced cellular clearance of damaged components.
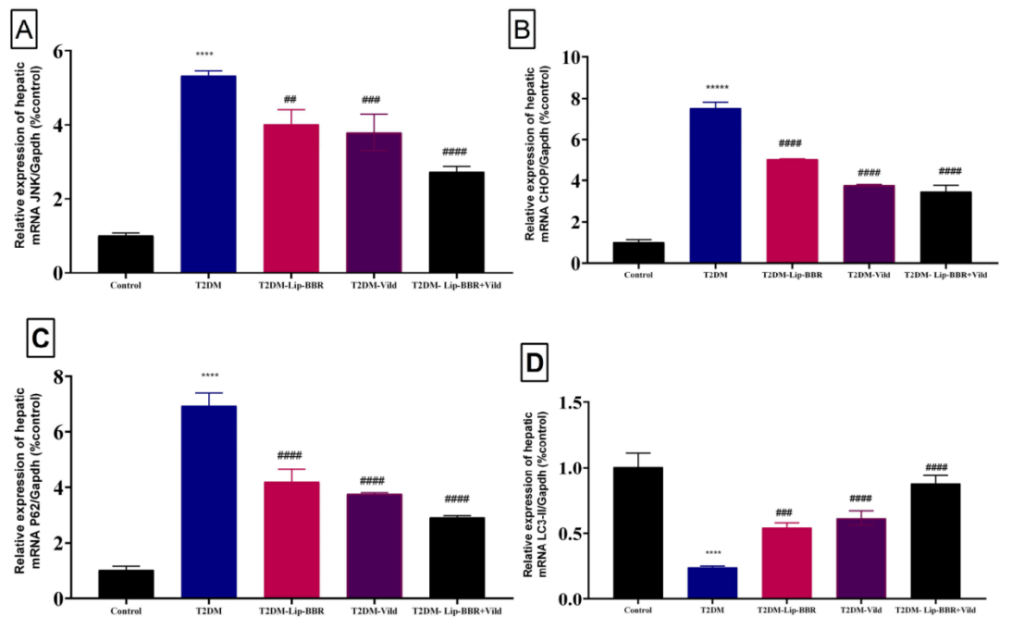
Significance: Autophagy plays a critical role in maintaining cellular homeostasis and reducing oxidative stress. The activation of this pathway suggests that Lip-BBR helps maintain liver function by promoting cellular repair mechanisms.
5. Metabolic and Biochemical Assessments
Procedure: The study evaluated several metabolic parameters, including fasting blood glucose levels, insulin sensitivity (via the HOMA-IR index), and liver enzyme levels (ALT and AST) to gauge liver function.
Result:
Lip-BBR treatment significantly reduced fasting blood glucose and improved insulin sensitivity compared to the diabetic control group.
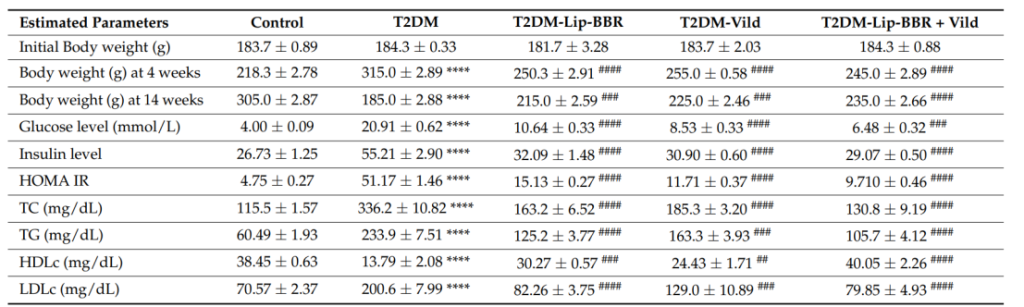
Liver enzymes (ALT and AST) were significantly reduced, indicating improved liver function.
Significance: These results highlight the metabolic benefits of Lip-BBR, demonstrating its ability to regulate glucose metabolism, reduce liver damage, and improve overall metabolic health in T2DM.
Conclusion
This study presents Lip-BBR as a promising therapeutic strategy for alleviating liver injury associated with Type 2 Diabetes Mellitus (T2DM). By enhancing autophagy and reducing oxidative stress, Lip-BBR addresses both metabolic dysfunctions and liver damage, offering a dual benefit for T2DM management. Furthermore, the liposomal formulation significantly improves the bioavailability of berberine, enhancing its therapeutic potential.
The research underscores the importance of developing innovative therapies that target both the liver and metabolic dysfunctions in T2DM. The combination of Lip-BBR and vildagliptin showed synergistic effects, suggesting that Lip-BBR could be an effective adjunct to existing diabetes treatments.
While the findings are promising, the study acknowledges that further clinical research, particularly human trials, is required to confirm the long-term safety and efficacy of Lip-BBR. However, the innovative nature of Lip-BBR, combined with its potential to improve liver function and metabolic regulation, paves the way for future breakthroughs in the treatment of diabetes and its complications.
Reference:
Khater, Safaa I., et al. “Liposome-encapsulated Berberine alleviates liver injury in type 2 diabetes via promoting AMPK/mTOR-mediated autophagy and reducing ER stress: morphometric and Immunohistochemical scoring.” Antioxidants 12.6 (2023): 1220.
