Editor: Nina
Key Preview
Research Question
- How can mRNA delivery systems be optimized to effectively transfect primary T lymphocytes and achieve selective organ tropism, while overcoming the limitations of existing delivery methods?
Research Design and Strategy
- Introduction of bAC CARTs: The study introduces CARTs with a novel beta-amido carbonate (bAC) backbone, designed to improve mRNA delivery efficiency and selectivity.
- Structural Modifications: The bAC backbone features distinct side-chain spacing and lipidic components, improving stability, transfection rates, and organ-specific targeting.
- Key Target: The research focuses on primary T lymphocytes, which are difficult to transfect but crucial for therapies like CAR-T cell engineering and genome editing.
Method
- Polymer Synthesis: bAC CARTs were synthesized using organocatalytic ring-opening polymerization. Variations in lipid and cationic block lengths were systematically tested.
- In Vitro Studies: Transfection efficiency was evaluated in T cells (Jurkat cells and primary lymphocytes) using eGFP mRNA and flow cytometry.
- In Vivo Tropism Evaluation: Mouse models were used to study spleen-specific delivery and the functional activity of transfected immune cells.
Key Results
- Achieved up to 70% T-cell transfection efficiency in vitro, surpassing previous CART systems.
- Demonstrated 97% selective spleen tropism for mRNA delivery in vivo, with significant transfection of T and B lymphocytes.
- Showed that bAC CARTs enable efficient CAR-T cell engineering and higher protein expression compared to existing delivery methods.
Significance of the Research
- Therapeutic Potential: bAC CARTs could revolutionize immunotherapy and genome editing by providing a safer, more effective delivery system.
- Selective Organ Targeting: Their spleen tropism minimizes off-target effects, paving the way for targeted treatments in immune modulation and cancer therapy.
- Broader Applications: Beyond mRNA, bAC CARTs hold potential for delivering other polyanions like siRNA and circular RNA (circRNA).
Overcoming Barriers in mRNA Delivery
Researchers have long grappled with the challenge of delivering mRNA effectively, particularly to primary T lymphocytes, which are crucial in immunotherapy and genome editing. Existing methods, like electroporation and lipid nanoparticles (LNPs), often face limitations such as inefficiency, off-target effects, and scalability issues. To address these, a recent study introduces a novel class of charge-altering releasable transporters (CARTs) with a beta-amido carbonate (bAC) backbone. These CARTs aim to revolutionize mRNA delivery by enhancing efficiency, specificity, and safety, particularly in immune cells like T lymphocytes.
Behind the Research
The study, published in Nature Communications, represents a collaboration between leading scientists from Stanford University. The team aimed to create a delivery system that bridges the gap between laboratory research and clinical application, focusing on scalability, safety, and specificity.

Key Experimental Procedures
Synthesis of bAC CARTs
Procedure: Developed 24 bAC CART variants using an economical, two-step polymerization process (organocatalytic ring-opening and deprotection).
Results: Created CARTs with unique lipid and cation block lengths, achieving nanoparticle sizes of 130–280 nm.
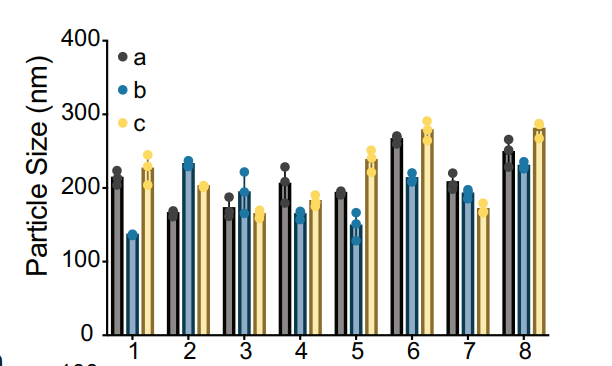
Significance: The new bAC backbone improved stability and enabled precise control of mRNA release.
In Vitro Transfection Efficiency
Procedure: T lymphocytes (Jurkat and primary cells) were transfected with eGFP mRNA using bAC CARTs, and transfection rates were quantified via flow cytometry.
Results: Achieved 70% transfection in Jurkat cells and up to 65% transfection in primary T cells, outperforming earlier CARTs and lipid-based systems.
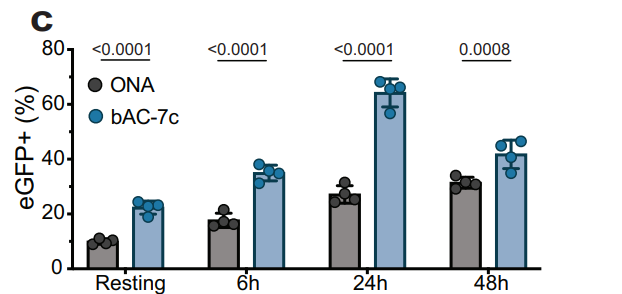
Significance: Demonstrated the efficacy of bAC CARTs in overcoming the challenges of primary T-cell transfection.
In Vivo Spleen Tropism Evaluation
Procedure: Delivered luciferase mRNA via bAC CARTs in mouse models and analyzed protein expression using bioluminescence imaging.
Results: Observed 97% spleen-specific mRNA uptake, with superior luciferase expression compared to earlier CARTs.
Significance: Highlighted the potential of bAC CARTs for organ-specific mRNA delivery, reducing off-target effects.
CAR-T Cell Engineering
Procedure: CD8+ T cells were transfected with anti-human CD19 CAR mRNA using bAC CARTs and co-cultured with leukemia cells (Nalm6-GL).
Results: Engineered CAR-T cells exhibited higher CD19-CAR expression and enhanced cytotoxic activity against target cells.
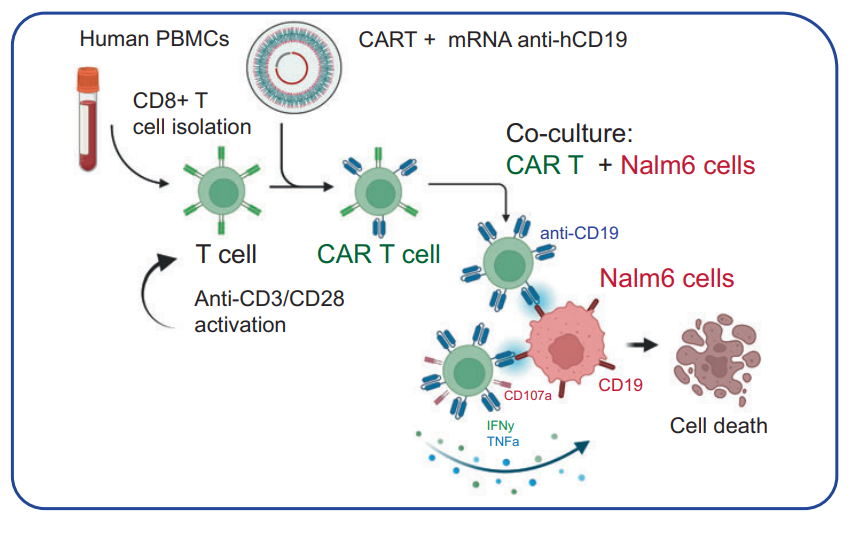
Significance: Validated bAC CARTs as an effective platform for CAR-T cell generation, critical for cancer immunotherapy.
Safety and Functional Analysis
Procedure: Monitored cell viability, toxicity, and functional markers (CD25, CD69, IFN-γ, TNF-α) post-transfection.
Results: bAC CARTs showed minimal toxicity, maintained T-cell functionality, and supported cytokine production.
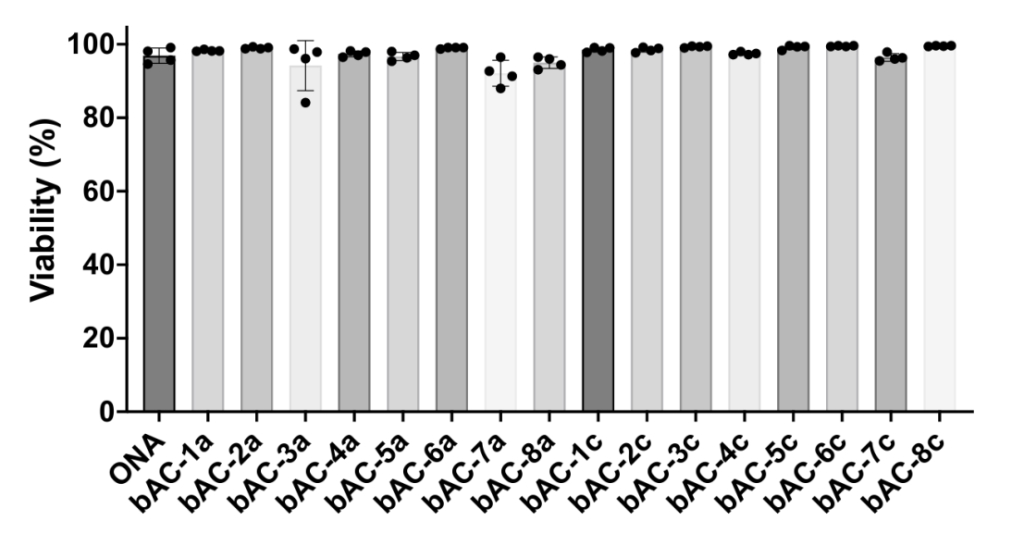
Significance: Demonstrated the safety and clinical viability of bAC CARTs.
Conclusion
This study underscores the potential of bAC CARTs as a game-changer in mRNA delivery. By enhancing transfection efficiency and ensuring organ-specific tropism, these CARTs pave the way for advanced therapeutic applications. As research progresses, the bAC system could become a cornerstone technology in the field of RNA-based therapies, addressing unmet needs in immunotherapy, genome editing, and beyond.
Reference:
Li, Zhijian, et al. “Charge-altering releasable transporters enhance mRNA delivery in vitro and exhibit in vivo tropism.” Nature Communications 14.1 (2023): 6983.
