Key Preview: Unraveling the Research Questions
In the realm of cancer therapeutics, a pivotal challenge remains: how to effectively deliver drugs to tumors that often possess tight endothelial barriers. The study at hand addresses this issue by investigating the ability of engineered gold nanoparticles, termed NanoEL (nanoparticle-induced endothelial leakiness), to induce vascular leakiness in tumor tissues. This research seeks to determine if these nanoparticles can facilitate enhanced drug delivery through controlled manipulation of tumor vasculature.
The methodology employed involved animal models with various types of tumors, wherein a library of gold nanoparticles was synthesized and characterized based on size and surface properties. Key aspects included an experimental design that allowed for real-time imaging and quantification of nanoparticle penetration into tumor interstitial spaces. Notably, pretreatment with NanoEL particles prior to therapeutic administration demonstrated significant efficiency improvements.
Quantitative results reveal that treatment with NanoEL particles led to a significant increase in permeability within tumor vasculature—reported increases were noted in both primary tumors and micrometastases, resulting in complete regression in several cases without exacerbating metastasis.
The findings underscore the potential implications for cancer therapy; notably, they suggest that engineering vascular leakiness could improve therapeutic access without increasing systemic toxicity. Limitations do exist; there remains a need for further exploration into long-term effects and optimal dosing regimens alongside suggestions for future research avenues focusing on diverse tumor types and combinations with existing therapies.
Journey Through Research Evolution
Over recent decades, cancer treatment has evolved from generalized cytotoxic approaches towards more targeted strategies aimed at overcoming biological barriers associated with tumors. Historically reliant on passive mechanisms such as the enhanced permeability and retention (EPR) effect, current methodologies are increasingly seeking active means—such as those proposed by this study—to improve drug delivery systems substantially.
This research introduces an innovative approach by utilizing engineered nanoparticles capable of transiently disrupting endothelial junctions specifically at tumoral sites—thereby enhancing accessibility while minimizing collateral damage typically inflicted upon healthy tissues. The central question revolves around whether such artificial modulation can be reliably achieved without adverse effects on normal physiology—a challenge this study directly confronts.
Objectives Behind Findings
The research team comprises Magdiel Inggrid Setyawati et al., who conducted their work between 2022-2023 across several esteemed institutions including National University of Singapore and University of Science and Technology of China before publishing their findings in Nature Communications.
Significance lies not only in advancing nanoparticle technology but also potentially revolutionizing the way cancer therapies are administered. The primary objective of this study was to demonstrate that engineered nanoparticles can induce vascular leakiness in tumors, thereby facilitating the enhanced delivery of anti-cancer drugs and improving patient outcomes.
Methodology: A Detailed Experimental Process
The researchers designed their studies utilizing a library of gold nanoparticles varying in size and surface properties to investigate their effects on endothelial cells. The experimental design included both in vitro assays with cultured endothelial monolayers and in vivo intravital imaging using murine models implanted with various types of tumors.
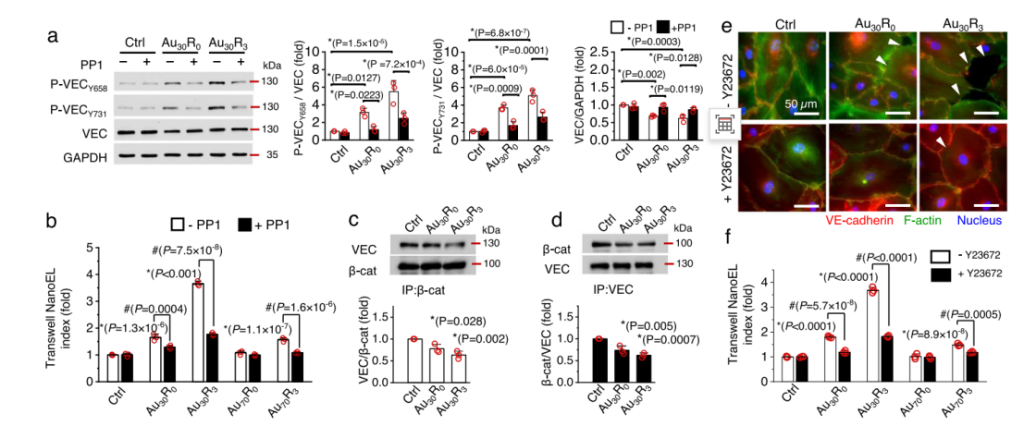
Fig.| Au NanoEL induction required activation of VE-cadherin signaling and actin remodeling process
Key procedures involved exposing the endothelial cell layers to different formulations of nanoparticles and observing the resultant changes in permeability. Additional techniques such as transwell assays allowed quantification of nanoparticle-induced leakiness, while real-time imaging provided dynamic insights into how these particles affect tumor vasculature over time.
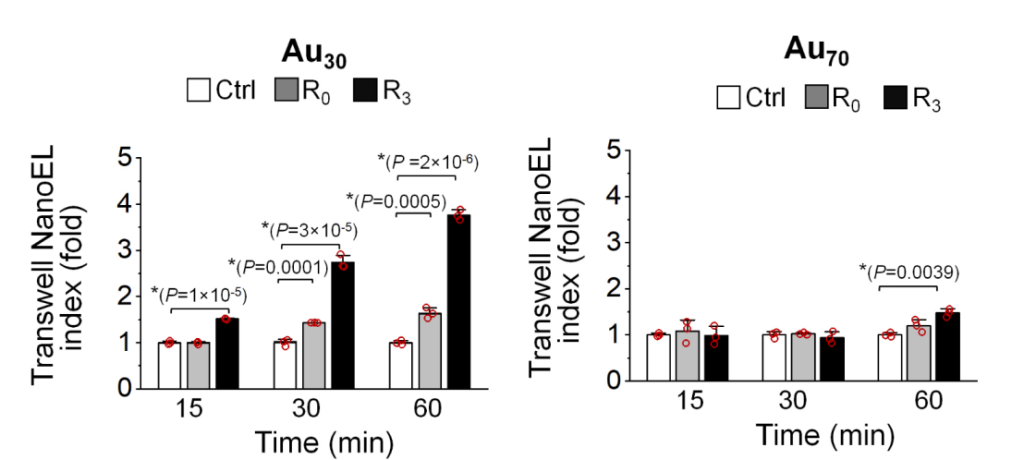
Fig| Induction of Au NanoEL occurred in time dependent manner.
Statistical analysis revealed that certain formulations notably increased intercellular gaps—ranging from nanometers to several micrometers—facilitating significant leakage into tumor tissues. Results indicated that nanoparticle treatment led to improved infiltration rates for larger therapeutic agents, showcasing their innovative application for drug delivery systems.
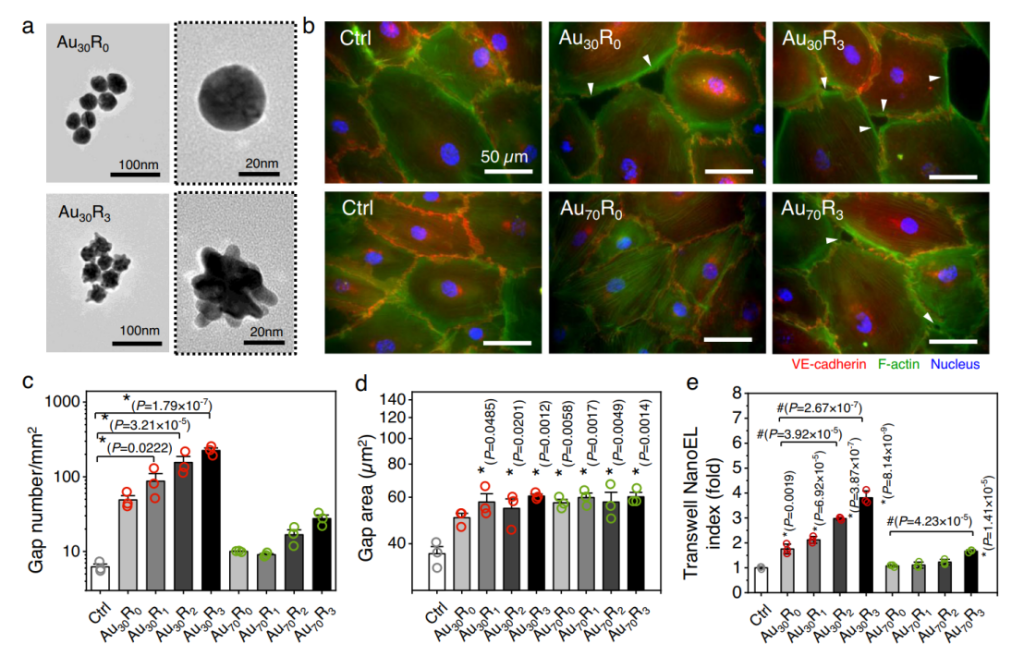
Fig| Au NPs induced endothelial cell leakiness (Au NanoEL) in size and surface
roughness dependent manner.
Conclusion: Implications and Future Directions
The study concludes with robust findings indicating that NanoEL particles effectively enhance vascular leakiness within tumors, allowing previously restricted therapeutic access. This innovation carries substantial implications; it could lead to more effective cancer treatments by ensuring higher concentrations of drugs reach target sites without substantially affecting healthy tissues elsewhere.
However, limitations were acknowledged regarding variations based on tumor microenvironment differences and the potential need for repeated dosing strategies. Moving forward, future research should explore broader applications across different cancer types, optimize nanoparticle characteristics further, and assess long-term effects on both efficacy and safety profiles within clinical settings.
In summary, this approach holds promise not only for improving existing therapies but also for paving new avenues towards more effective cancer care through engineered biomedical technologies.
Reference:
Setyawati, Magdiel Inggrid, et al. “Engineering tumoral vascular leakiness with gold nanoparticles.” Nature Communications 14.1 (2023): 4269.
