Key Preview: A Promising Solution for Precision Cancer Therapy
Researchers have developed an innovative multifunctional bioresponsive and fluorescent-active nanogel designed for both breast cancer therapy and bioimaging. The study addresses the need for more precise, effective treatments for HER2-positive breast cancer, a particularly aggressive form of the disease. The proposed nanogel not only delivers therapeutic agents efficiently but also enhances imaging capabilities, providing a theranostic (therapeutic and diagnostic) solution.
The research team synthesized the nanogel by integrating creatinine-based carbon dots (CDs) into a lecithin-inulin matrix. The nanogel was PEGylated to improve its stability and functionality, and it was conjugated with herceptin, a well-known monoclonal antibody that targets HER2 receptors. The system was rigorously tested on HER2-positive breast cancer cells and further evaluated in mouse models to confirm its effectiveness.
Key results showed that the nanogel effectively delivered herceptin to cancer cells, induced significant apoptosis (programmed cell death), inhibited tumor growth in vivo, and enhanced imaging precision, all while maintaining biocompatibility. These findings highlight the nanogel’s potential as a powerful platform for targeted cancer therapy, although further studies are required for clinical application.
The Evolution of Cancer Therapy and Research Innovation
Breast cancer remains a leading cause of cancer-related deaths globally. Over the past two decades, targeted therapies such as herceptin (trastuzumab) have significantly improved outcomes for HER2-positive patients by blocking the HER2 receptors that drive tumor growth. Despite this progress, challenges persist, including treatment toxicity, limited bioavailability, and insufficient imaging techniques for monitoring therapy response.
To address these issues, the integration of nanotechnology into cancer treatment has opened new avenues. Nanomaterials, such as carbon dots and polymeric nanogels, offer precise drug delivery and real-time imaging capabilities, advancing both diagnosis and therapy. This study combines these strengths by integrating herceptin with a PEGylated lecithin-inulin nanogel loaded with fluorescent carbon dots. This innovation not only enhances targeted drug delivery but also facilitates imaging to track tumor progression and treatment efficacy.
The research focuses on improving HER2-targeted therapies while minimizing off-target toxicity and ensuring efficient imaging, a critical step toward personalized cancer care.
Research Objective: Developing a Multifunctional Nanocarrier
The study was conducted by an interdisciplinary research team led by experts from Damghan University, Shahid Chamran University, and other international institutes. The findings were published in Advanced Composites and Hybrid Materials in 2022.
The primary objective was to design and evaluate a bioresponsive and fluorescent-active nanogel that integrates herceptin for targeted delivery and bioimaging. The research aimed to improve therapeutic outcomes for HER2-positive breast cancer while providing an imaging solution to monitor treatment effectiveness.
Innovative Experimental Design and Process
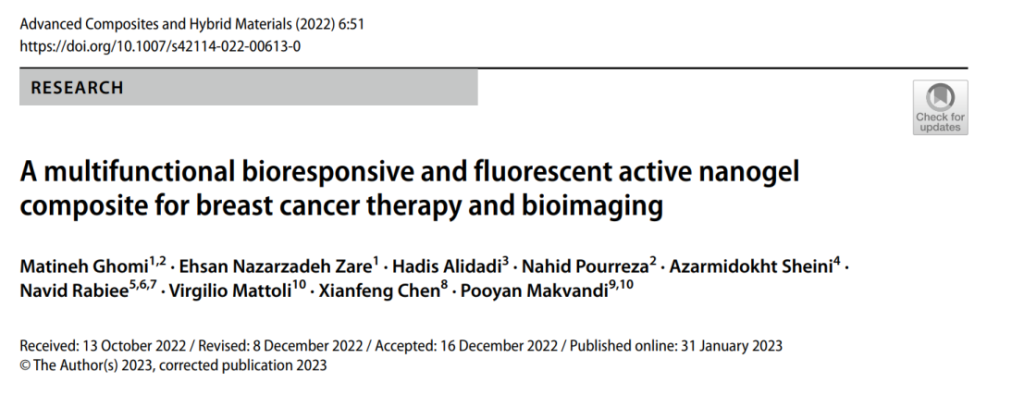
The research followed a comprehensive approach, starting with the synthesis of carbon dots using a one-step hydrothermal reflux method. These carbon dots, known for their fluorescent properties and biocompatibility, were embedded into a lecithin-inulin nanogel using a water-in-oil nanoemulsion technique. The nanogel was further functionalized through PEGylation, a process that enhances its stability, reduces immunogenicity, and prolongs circulation time in the body. Finally, herceptin was conjugated to the nanogel to enable targeted delivery to HER2-positive cancer cells.
Characterization techniques, including X-ray diffraction (XRD), transmission electron microscopy (TEM), and Fourier transform infrared spectroscopy (FTIR), were used to confirm the nanogel’s structure and properties. Dynamic light scattering (DLS) measurements confirmed the nanogel’s optimal size (~100 nm), ensuring effective cellular uptake.
Key experiments included:
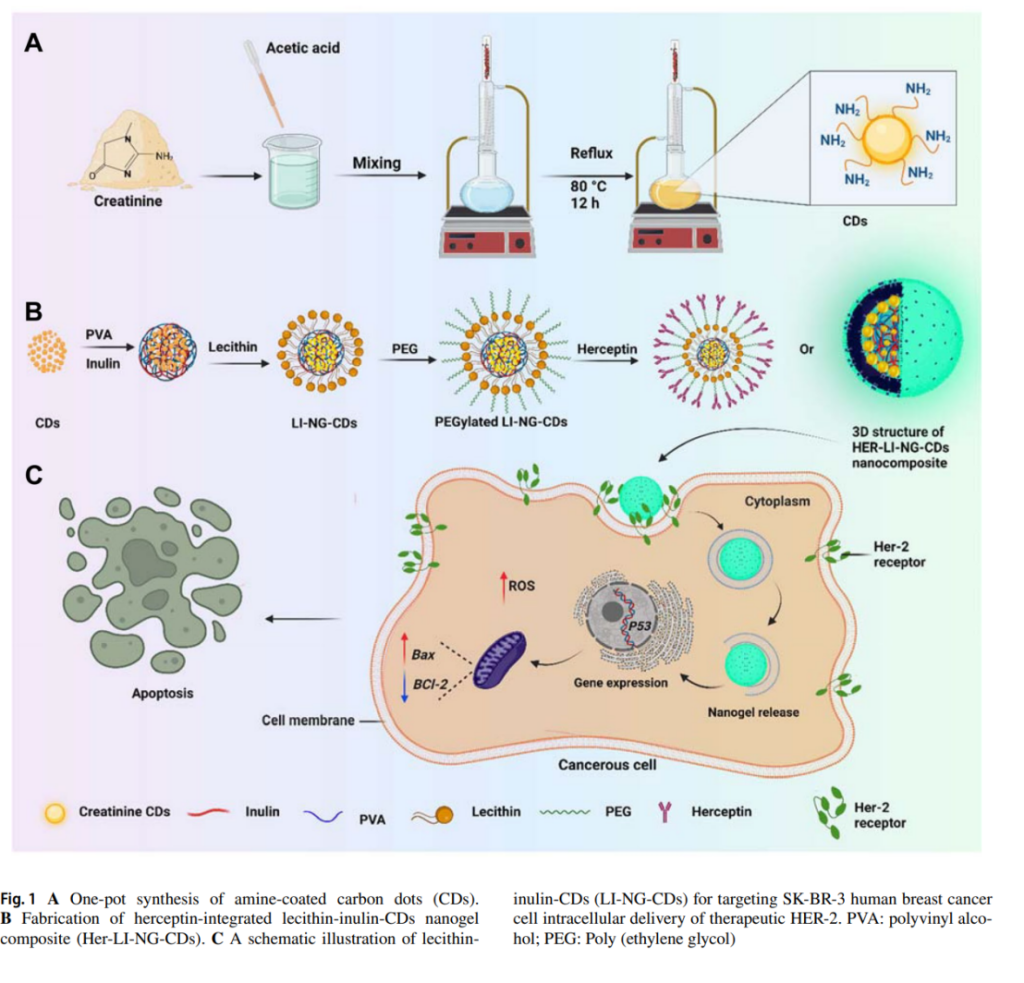
- In Vitro Studies: The nanogel’s performance was tested on HER2-positive (SK-BR-3) and triple-negative (MDA-MB-231) breast cancer cell lines. Results showed selective cytotoxicity against SK-BR-3 cells, with reduced ROS (reactive oxygen species) levels and increased apoptosis.
- Gene Expression Analysis: The nanogel upregulated apoptotic markers (Bax and p53) while downregulating anti-apoptotic markers (Bcl-2), confirming its role in inducing cell death.
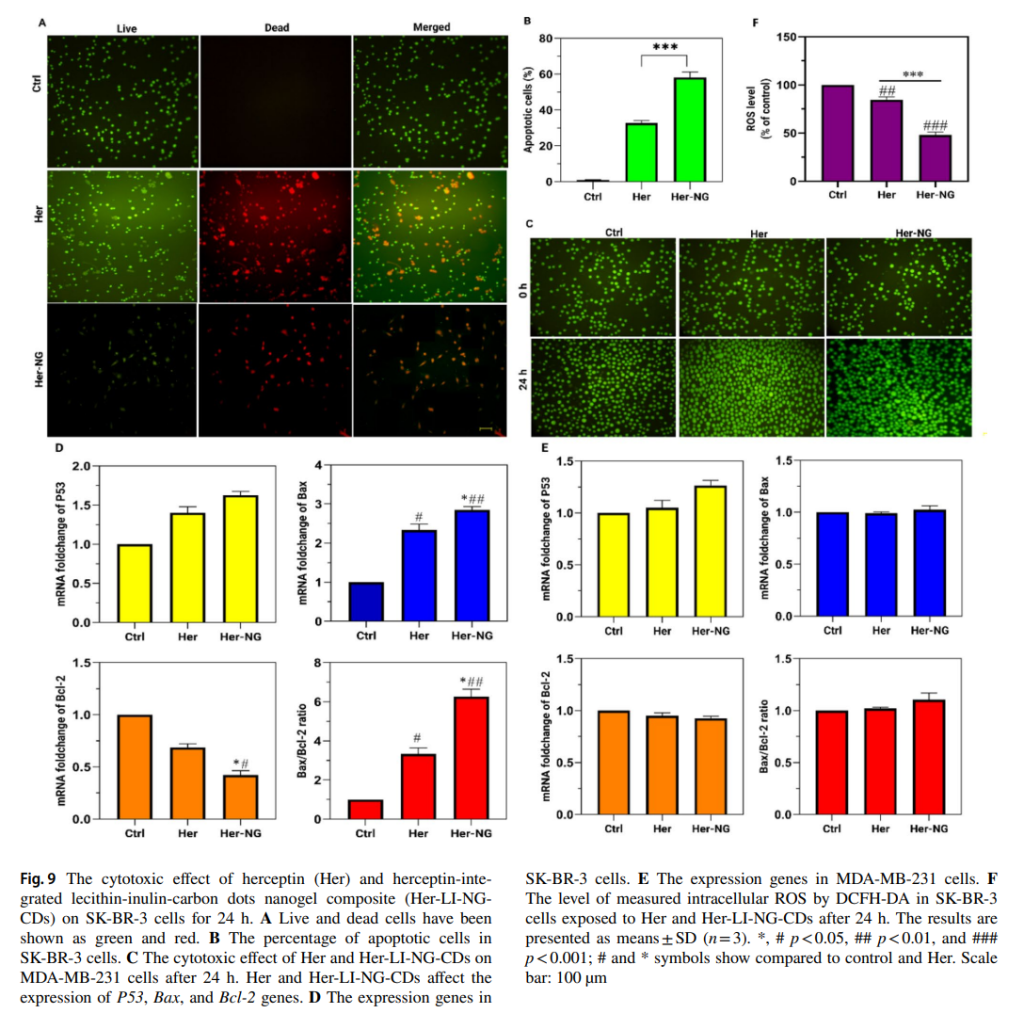
- In Vivo Testing: Mouse models injected with the nanogel demonstrated significant tumor growth inhibition and maintained normal tissue integrity, indicating the system’s biocompatibility and therapeutic efficacy.
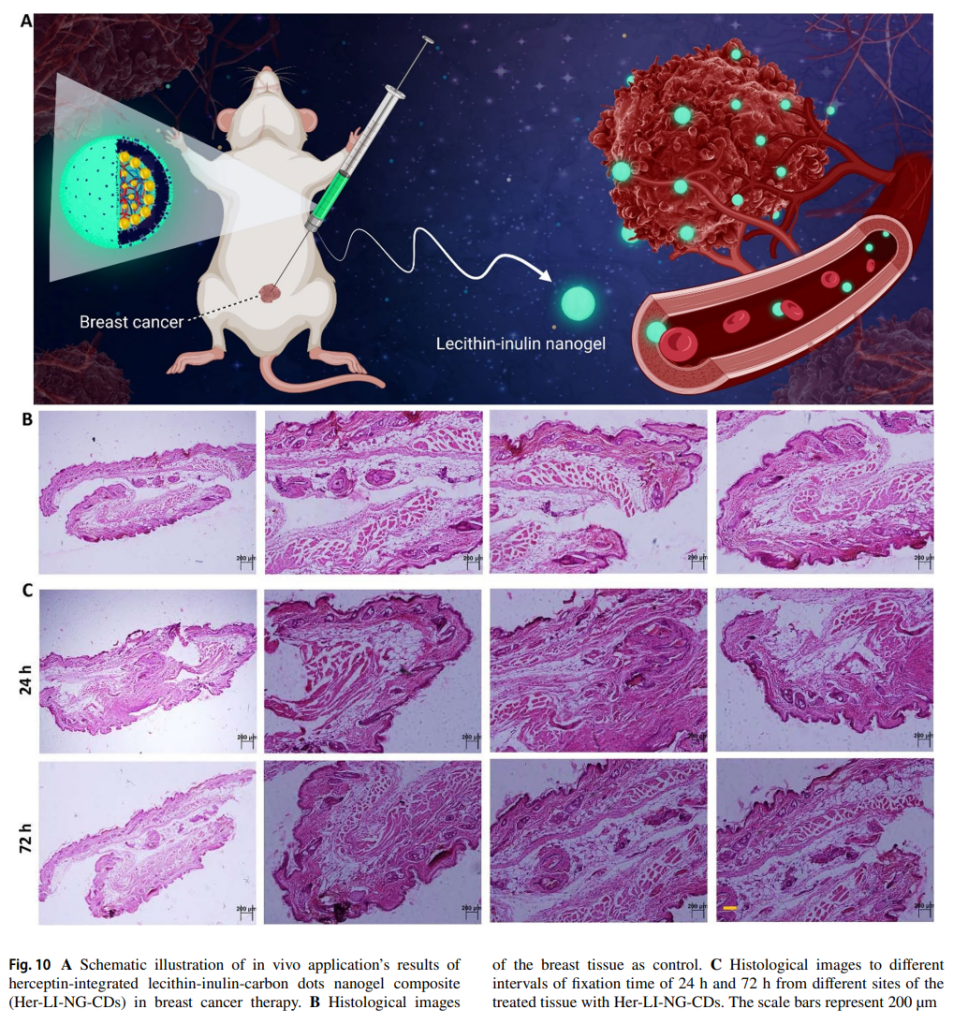
These results validated the nanogel’s ability to deliver herceptin efficiently while enhancing imaging capabilities due to the fluorescence of carbon dots.
Conclusion: Toward a New Era in Cancer Therapy
This study presents a significant step forward in targeted breast cancer treatment and imaging. The herceptin-integrated, PEGylated nanogel successfully demonstrated enhanced drug delivery, improved cellular uptake, and real-time imaging capabilities. Key findings include:
- Effective inhibition of HER2-positive breast cancer cell growth.
- Induction of apoptosis through targeted delivery.
- Tumor growth suppression in animal models.
- Real-time imaging enabled by fluorescent carbon dots.
The implications of this research are profound. By combining therapy and imaging into a single platform, the nanogel offers a promising theranostic solution for breast cancer treatment, addressing current limitations in drug delivery and monitoring.
However, further studies are necessary to optimize the nanogel’s dosage, evaluate its long-term safety, and explore its applications in other cancer types. Future research should focus on clinical translation to ensure this technology can benefit patients in real-world settings.
Reference:
Ghomi, Matineh, et al. “A multifunctional bioresponsive and fluorescent active nanogel composite for breast cancer therapy and bioimaging.” Advanced Composites and Hybrid Materials 6.1 (2023): 51.
