Introduction: A New Dawn for Light-Activated Drug Delivery
Advancements in cancer therapy have witnessed a paradigm shift with the emergence of light-activated drug delivery systems, combining precision with minimized side effects. Building upon these innovations, the latest study leverages metallophilic self-assembly to create a palladium-based light-activated anticancer drug. This breakthrough addresses key challenges of traditional chemotherapy, including nonspecific targeting, rapid clearance, and systemic toxicity, which often hinder treatment efficacy and patient outcomes. By harnessing supramolecular interactions, this research introduces a new class of drug delivery systems capable of self-assembling in vivo to target tumors with unparalleled precision.
Research Objective: Collaborative Efforts to Redefine Therapy

This groundbreaking research is the result of a global collaboration involving esteemed contributors such as Xue-Quan Zhou, Peiyuan Wang, Sylvestre Bonnet, and their multidisciplinary team from institutions like Dalian University of Technology, Leiden University, and Johns Hopkins University. Published in Nature Chemistry, the study sets its sights on revolutionizing cancer treatment by addressing inefficiencies in traditional nanocarriers. The research focuses on developing a palladium-based self-delivery drug system that not only ensures high tumor accumulation but also exhibits exceptional photodynamic therapy (PDT) efficacy.
Experimental Process: From Synthesis to Groundbreaking Results
The research team designed a palladium-based drug (PdL) using an innovative synthesis approach, leveraging bis-cyclometalated palladium compounds. These compounds exhibit unique metallophilic interactions, enabling them to self-assemble into stable nanoparticles in physiological environments. The synthesis involved a two-step process: first, the creation of a ligand precursor, followed by coordination with palladium to form PdL. Advanced analytical techniques such as dynamic light scattering, cryogenic electron microscopy, and X-ray crystallography were employed to confirm the structural integrity and self-assembly properties of PdL.
Once introduced into biological media, PdL molecules spontaneously formed nanoparticles with sizes averaging 181 nm, maintaining stability in complex biological conditions such as blood circulation. In vivo experiments demonstrated that these nanoparticles accumulate efficiently in tumor tissues, achieving a remarkable tumor uptake rate of 10.2% of the injected dose per gram of tissue—far exceeding conventional nanocarriers.
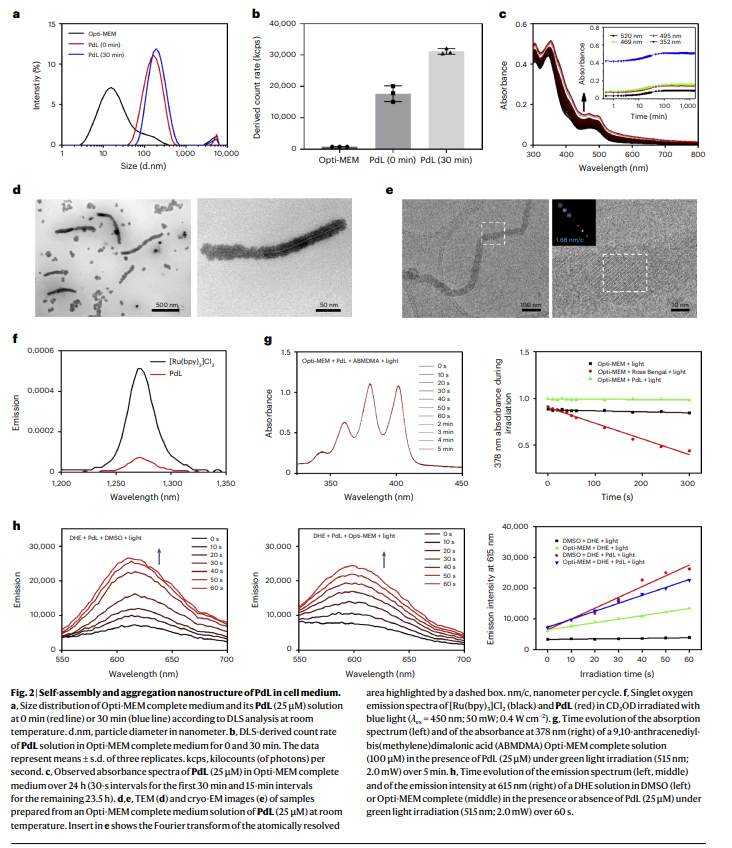
The study also highlighted the nanoparticles’ exceptional photodynamic efficacy. Upon activation with green light, PdL produced superoxide radicals through a Type I photodynamic mechanism, leading to targeted tumor destruction. Photodynamic therapy experiments revealed a 96-fold increase in cytotoxicity after light activation, demonstrating the system’s capacity for precise cancer cell eradication while minimizing damage to surrounding healthy tissues. The PdL nanoparticles exhibited prolonged circulation in the bloodstream (up to 12 hours), further enhancing their tumor-targeting efficiency.
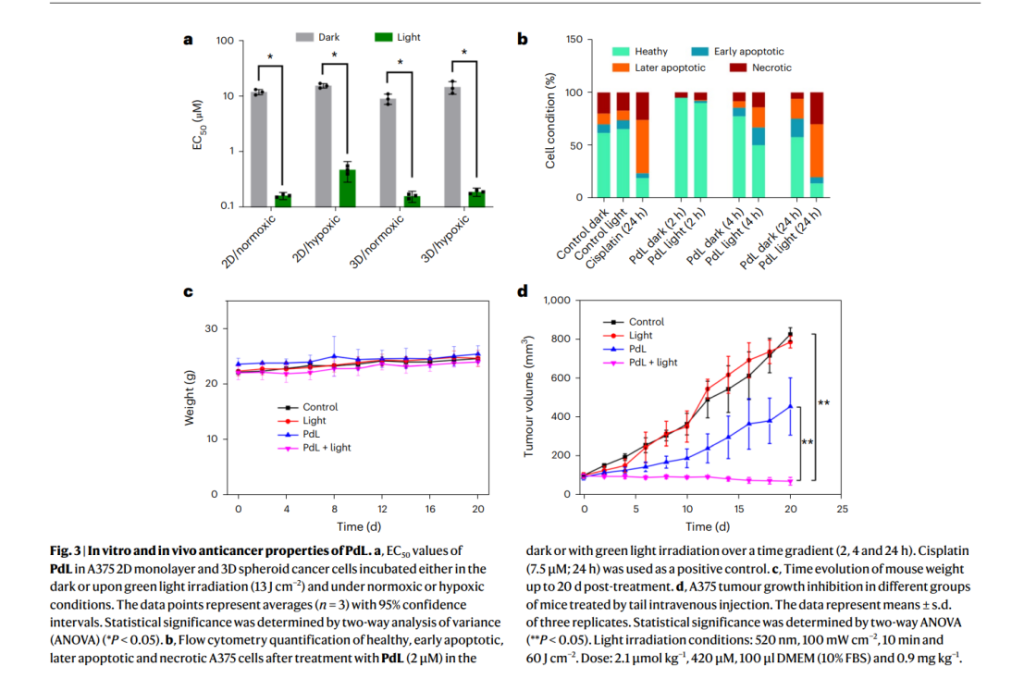
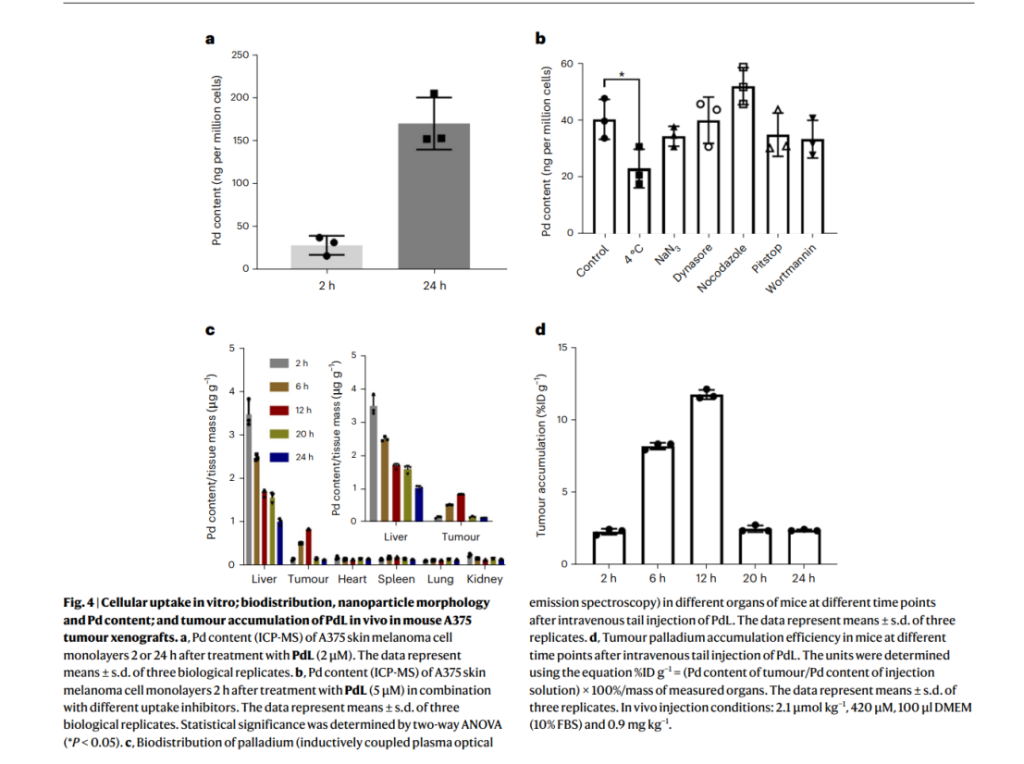
These results underscore the dual advantages of PdL: its ability to self-assemble into drug-loaded nanoparticles in vivo and its remarkable photodynamic activity, setting a new standard for light-activated cancer therapies.
Conclusion: Toward a Brighter Future in Cancer Treatment
This study marks a significant leap in cancer nanomedicine, showcasing the potential of metallophilic self-assembly to address longstanding challenges in drug delivery. By combining high tumor specificity with low systemic toxicity, PdL offers a promising alternative to traditional chemotherapy and nanocarrier systems. The research underscores the transformative impact of light-activated therapies, paving the way for more patient-friendly cancer treatments.
While the findings are promising, the study acknowledges limitations, including the dependency on light activation and challenges in penetrating deeper tumor sites. Future research will focus on enhancing the specificity and versatility of light-activated drug systems, aiming to expand their applications across various cancer types and treatment settings.
Reference:
Zhou, Xue-Quan, et al. “In vivo metallophilic self-assembly of a light-activated anticancer drug.” Nature Chemistry 15.7 (2023): 980-987.
