A Historical Challenge: Tackling Chronic Wounds
Diabetic wounds, often plagued by biofilm infections and persistent inflammation, have long posed a challenge to the medical field. Traditional treatments—ranging from aggressive debridement to bioengineered tissues—have struggled to address the complex microenvironment of these wounds. Recent research emphasizes the critical role of this microenvironment in wound healing, particularly its interplay with reactive species and immune responses.
The study under discussion introduces a groundbreaking solution: a self-enhancing photodynamic immunomodulatory microneedle (MN) bandage. This innovative technology not only disrupts biofilm barriers but also regulates reactive species to promote wound healing. Such advancements mark a significant step forward in addressing the limitations of existing therapies and highlight the necessity of multifaceted approaches to combat chronic wounds effectively.
Driving Innovation: The Vision Behind the Research
Conducted by a multidisciplinary team from Sun Yat-sen University (China) and Nanyang Technological University (Singapore), the research culminated in its publication in Nature Communications in October 2023. Led by prominent scientists Li Yang, Dan Zhang, and Xiaowei Zeng, the team aimed to create a therapeutic solution that integrates antibacterial, anti-inflammatory, and pro-angiogenic properties to enhance diabetic wound healing.
The microneedle bandage leverages dopamine-coated hybrid nanoparticles containing selenium and chlorin e6 (SeC@PA). By targeting the unique biofilm microenvironment, this bandage introduces a novel dual-directional approach to reactive species regulation, showcasing its potential as a transformative tool for diabetic wound therapy.
Experimental Process: From Design to Discovery
The creation of the SeC@PA microneedle (MN) bandage followed a meticulously integrated approach, blending innovative design with comprehensive experimental evaluation. This multifunctional bandage was tailored to tackle the dual challenges of biofilm eradication and diabetic wound healing.
Design and Development
Researchers synthesized hybrid nanoparticles comprising selenium and chlorin e6, coated with dopamine (SeC@PA). These nanoparticles were engineered to respond to the unique microenvironment of chronic wounds, particularly the high-glutathione (GSH) levels in biofilms and low-GSH levels in inflamed tissues. The SeC@PA nanoparticles were loaded into dissolvable microneedles to form a bandage capable of penetrating wound barriers, delivering therapeutic agents directly to the affected sites, and dissolving upon application for optimal drug efficacy.
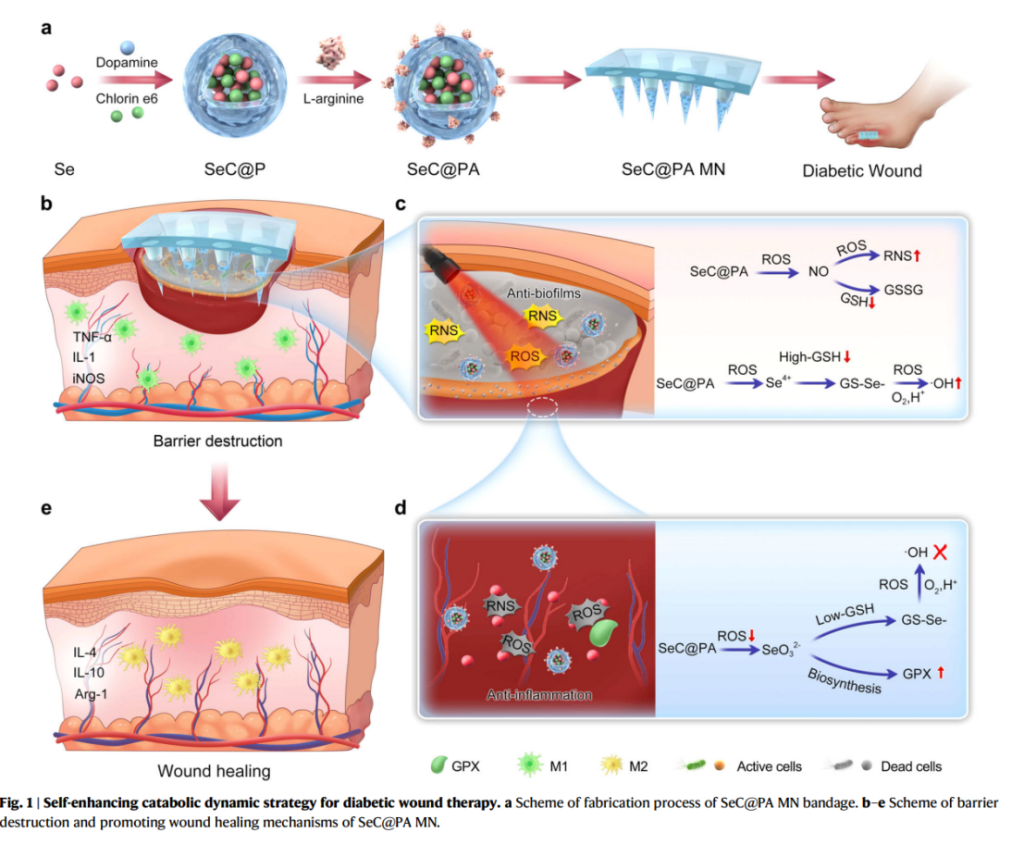
Biofilm Targeting and Oxidative Stress Modulation
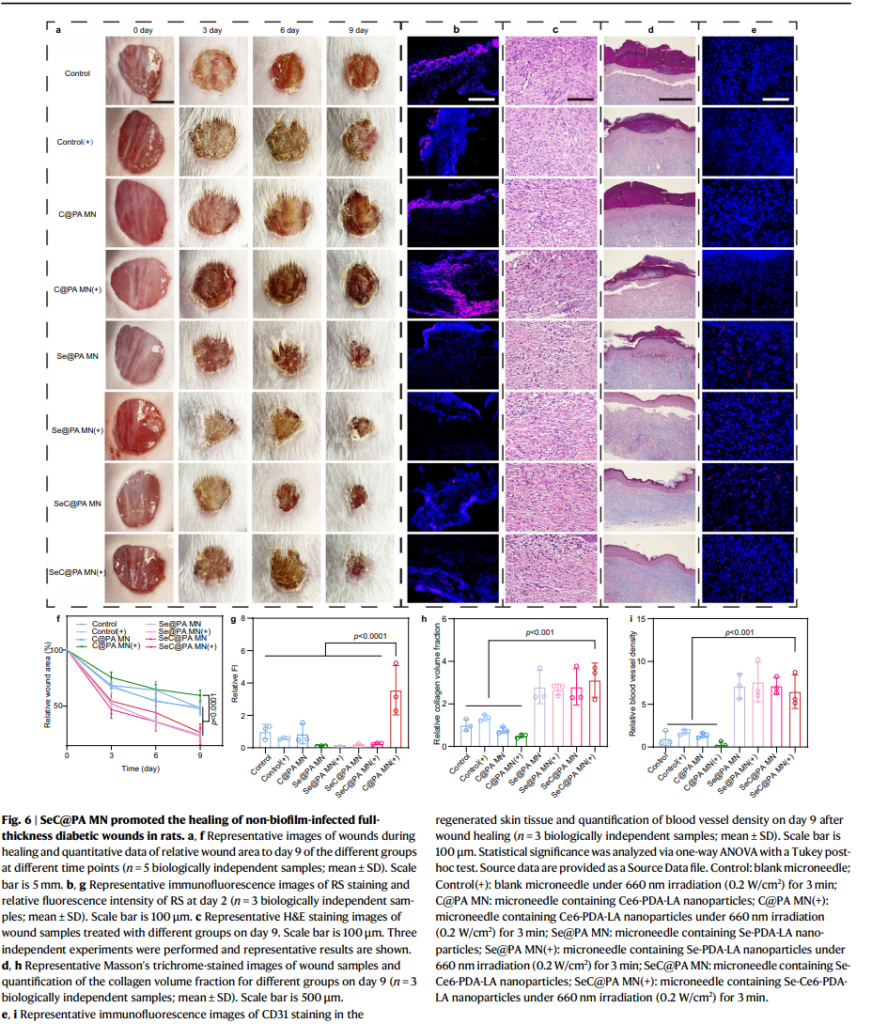
The bandage was tested in both in vitro and in vivo models, demonstrating remarkable efficiency in disrupting biofilm integrity and managing oxidative stress. The microneedles generated reactive oxygen and nitrogen species (ROS and RNS) within high-GSH biofilms, eradicating up to 95% of biofilms. Conversely, in inflamed wound tissues with low GSH levels, the bandage scavenged RS, mitigating oxidative damage and reducing inflammation.
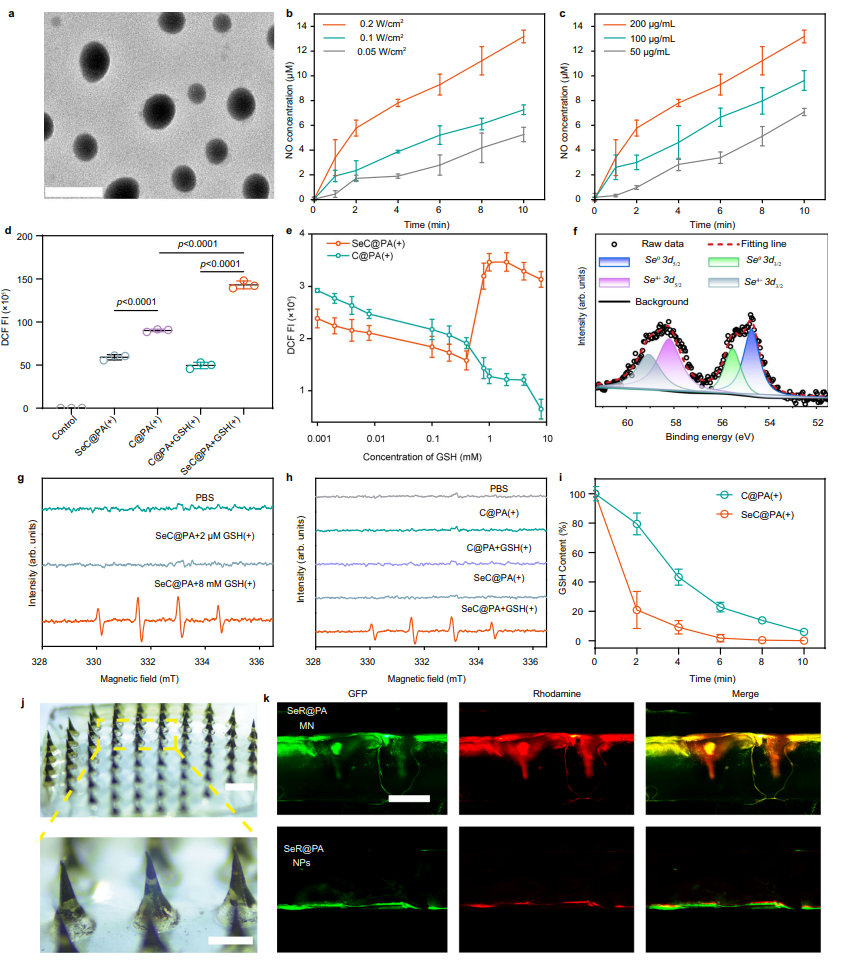
Immune Response and Tissue Regeneration
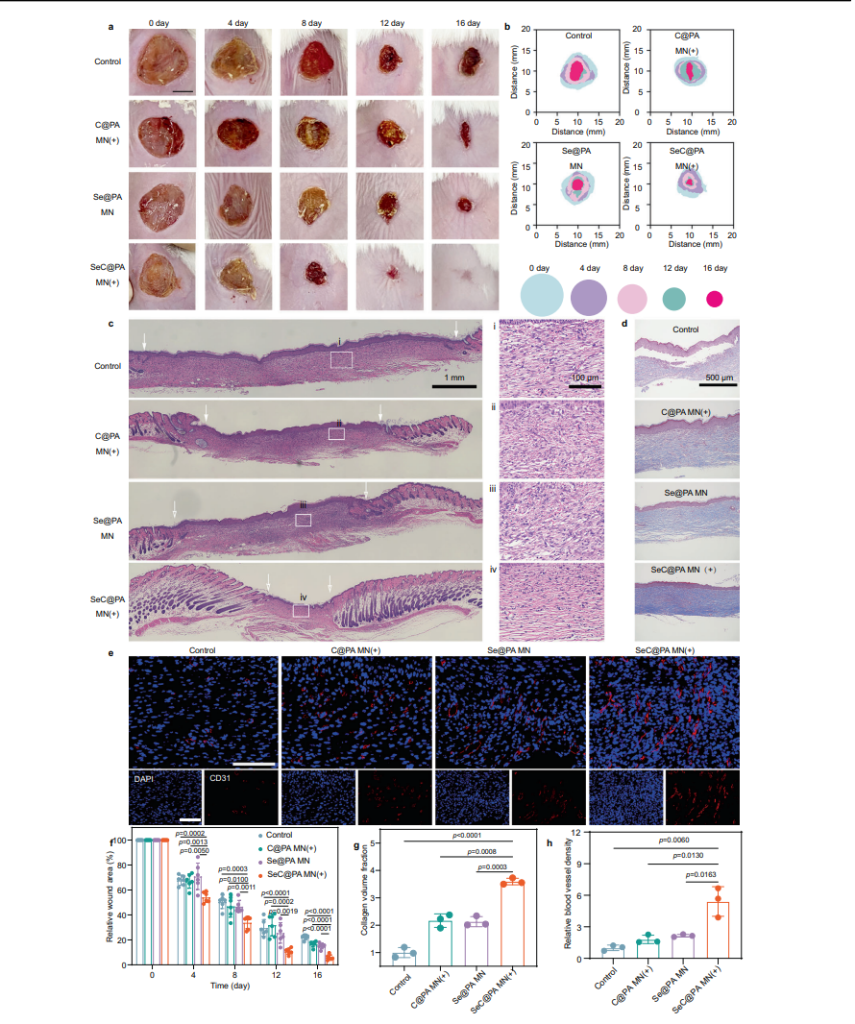
To promote healing, the SeC@PA MN bandage enhanced macrophage polarization toward the M2 phenotype, which plays a crucial role in reducing inflammation and supporting tissue repair. This immune modulation was accompanied by increased secretion of vascular endothelial growth factor (VEGF), driving angiogenesis and accelerating collagen deposition in the wound bed. In diabetic mouse models, the treatment improved blood vessel density and collagen deposition, significantly enhancing the wound healing process.
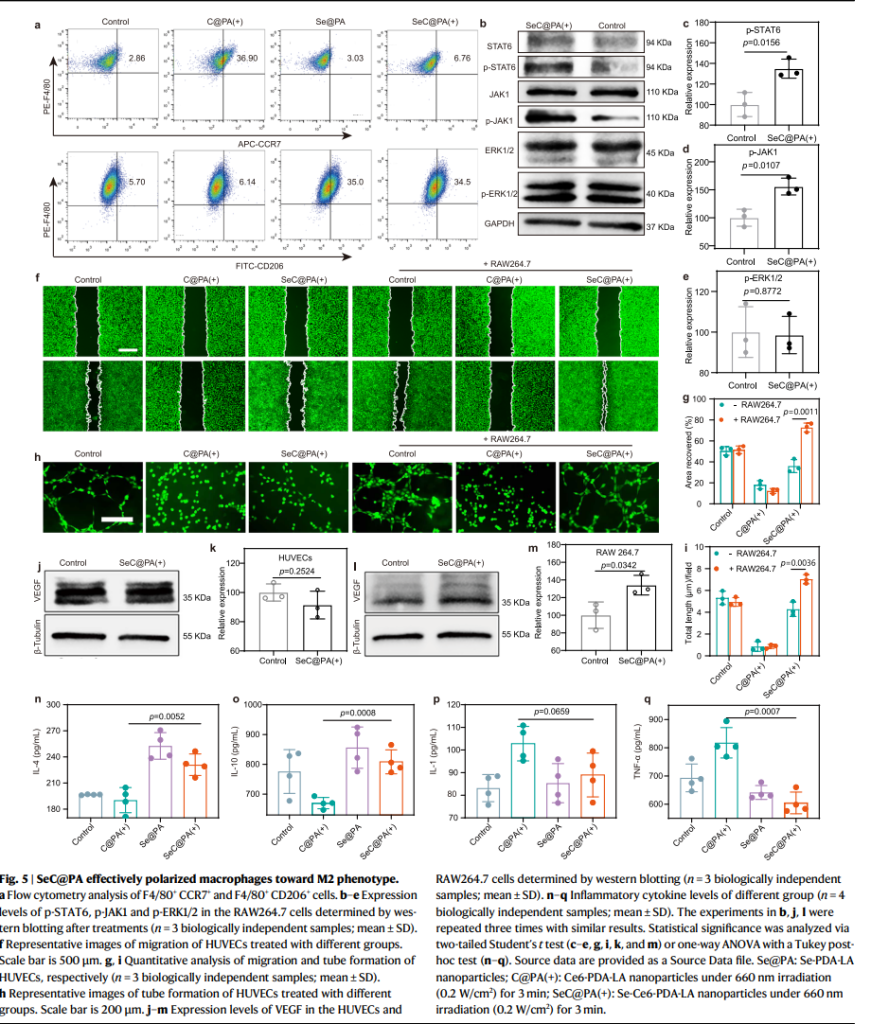
Therapeutic Efficacy in Wound Models
In diabetic mouse wound models, the bandage achieved nearly complete healing (95%) within 16 days, outperforming both untreated and conventionally treated groups. Its ability to penetrate biofilm barriers and deliver dual-action therapy to both biofilm-infected and inflamed tissues was key to its success.
This experimental journey underscores the transformative potential of the SeC@PA MN bandage in addressing the complex challenges of chronic wound care, paving the way for more effective and accessible treatments.
Transforming Care: Implications and Future Directions
This research underscores the transformative potential of multifunctional microneedle bandages in diabetic wound care. By addressing the interplay between biofilms, inflammation, and tissue regeneration, the SeC@PA bandage offers a holistic therapeutic strategy.
However, challenges remain. Translating these findings to clinical settings requires further investigation into large-scale manufacturing, long-term safety, and patient-specific adaptations. The study’s authors emphasize the need for future research to refine the technology and explore its applications beyond diabetic wounds, potentially extending to other chronic wound types.
Closing the Gap in Diabetic Wound Therapy
The SeC@PA microneedle bandage represents a significant leap in addressing diabetic wounds, combining innovative science with practical application. With its ability to tackle biofilm challenges, modulate immune responses, and enhance healing, this breakthrough technology paves the way for more effective and accessible treatments, offering new hope for patients worldwide.
Reference:
Yang, Li, et al. “Biofilm microenvironment triggered self-enhancing photodynamic immunomodulatory microneedle for diabetic wound therapy.” Nature Communications 14.1 (2023): 7658.
